Smad7 Antisense Oligonucleotide in Crohn’s Disease: A Re-Evaluation and Explanation for the Discordant Results of Clinical Trials
Abstract
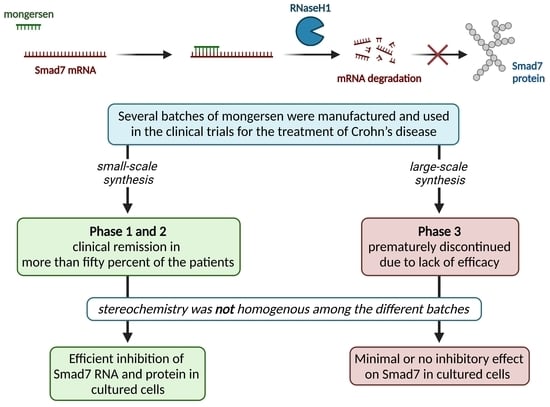
:1. Introduction
2. Clinical Trials of Mongersen in IBD
3. Loss in Bioactivity for Batches of Mongersen Used in Trials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacDonald, T.T.; Monteleone, I.; Fantini, M.C.; Monteleone, G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology 2011, 140, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef] [Green Version]
- Marafini, I.; Sedda, S.; Dinallo, V.; Monteleone, G. Inflammatory cytokines: From discoveries to therapies in IBD. Expert Opin. Biol. Ther. 2019, 19, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Bawardy, B.; Shivashankar, R.; Proctor, D.D. Novel and Emerging Therapies for Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 651415. [Google Scholar] [CrossRef]
- Macdonald, T.T.; Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 2005, 307, 1920–1925. [Google Scholar] [CrossRef] [Green Version]
- Hooper, L.V.; Macpherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef]
- Kuhn, R.; Lohler, J.; Rennick, D.; Rajewsky, K.; Muller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Pigneur, B.; Escher, J.; Elawad, M.; Lima, R.; Buderus, S.; Kierkus, J.; Guariso, G.; Canioni, D.; Lambot, K.; Talbotec, C.; et al. Phenotypic characterization of very early-onset IBD due to mutations in the IL10, IL10 receptor alpha or beta gene: A survey of the Genius Working Group. Inflamm. Bowel Dis. 2013, 19, 2820–2828. [Google Scholar] [CrossRef]
- Shull, M.M.; Ormsby, I.; Kier, A.B.; Pawlowski, S.; Diebold, R.J.; Yin, M.; Allen, R.; Sidman, C.; Proetzel, G.; Calvin, D.; et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 1992, 359, 693–699. [Google Scholar] [CrossRef]
- Guerrerio, A.L.; Frischmeyer-Guerrerio, P.A.; Huang, C.; Wu, Y.; Haritunians, T.; McGovern, D.P.B.; MacCarrick, G.L.; Brant, S.R.; Dietz, H.C. Increased Prevalence of Inflammatory Bowel Disease in Patients with Mutations in Genes Encoding the Receptor Subunits for TGFbeta. Inflamm. Bowel Dis. 2016, 22, 2058–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteleone, G.; Kumberova, A.; Croft, N.M.; McKenzie, C.; Steer, H.W.; MacDonald, T.T. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J. Clin. Investig. 2001, 108, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Pallone, F.; MacDonald, T.T. Smad7 in TGF-beta-mediated negative regulation of gut inflammation. Trends Immunol. 2004, 25, 513–517. [Google Scholar] [CrossRef]
- Boirivant, M.; Pallone, F.; Di Giacinto, C.; Fina, D.; Monteleone, I.; Marinaro, M.; Caruso, R.; Colantoni, A.; Palmieri, G.; Sanchez, M.; et al. Inhibition of Smad7 with a specific antisense oligonucleotide facilitates TGF-beta1-mediated suppression of colitis. Gastroenterology 2006, 131, 1786–1798. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Abdollah, S.; Qiu, Y.; Cai, J.; Xu, Y.Y.; Grinnell, B.W.; Richardson, M.A.; Topper, J.N.; Gimbrone, M.A., Jr.; Wrana, J.L.; et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell 1997, 89, 1165–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakao, A.; Afrakhte, M.; Moren, A.; Nakayama, T.; Christian, J.L.; Heuchel, R.; Itoh, S.; Kawabata, M.; Heldin, N.E.; Heldin, C.H.; et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature 1997, 389, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Izzo, R.; Bevivino, G.; De Simone, V.; Sedda, S.; Monteleone, I.; Marafini, I.; Di Giovangiulio, M.; Rizzo, A.; Franze, E.; Colantoni, A.; et al. Knockdown of Smad7 With a Specific Antisense Oligonucleotide Attenuates Colitis and Colitis-Driven Colonic Fibrosis in Mice. Inflamm. Bowel Dis. 2018, 24, 1213–1224. [Google Scholar] [CrossRef]
- Garo, L.P.; Ajay, A.K.; Fujiwara, M.; Beynon, V.; Kuhn, C.; Gabriely, G.; Sadhukan, S.; Raheja, R.; Rubino, S.; Weiner, H.L.; et al. Smad7 Controls Immunoregulatory PDL2/1-PD1 Signaling in Intestinal Inflammation and Autoimmunity. Cell Rep. 2019, 28, 3353–3366.e5. [Google Scholar] [CrossRef] [Green Version]
- Monteleone, G.; Fantini, M.C.; Onali, S.; Zorzi, F.; Sancesario, G.; Bernardini, S.; Calabrese, E.; Viti, F.; Monteleone, I.; Biancone, L.; et al. Phase I clinical trial of Smad7 knockdown using antisense oligonucleotide in patients with active Crohn’s disease. Mol. Ther. J. Am. Soc. Gene Ther. 2012, 20, 870–876. [Google Scholar] [CrossRef] [Green Version]
- Monteleone, G.; Neurath, M.F.; Ardizzone, S.; Di Sabatino, A.; Fantini, M.C.; Castiglione, F.; Scribano, M.L.; Armuzzi, A.; Caprioli, F.; Sturniolo, G.C.; et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N. Engl. J. Med. 2015, 372, 1104–1113. [Google Scholar] [CrossRef]
- Zorzi, F.; Calabrese, E.; Monteleone, I.; Fantini, M.; Onali, S.; Biancone, L.; Pallone, F.; Monteleone, G. A phase 1 open-label trial shows that smad7 antisense oligonucleotide (GED0301) does not increase the risk of small bowel strictures in Crohn’s disease. Aliment. Pharmacol. Ther. 2012, 36, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Di Sabatino, A.; Ardizzone, S.; Pallone, F.; Usiskin, K.; Zhan, X.; Rossiter, G.; Neurath, M.F. Impact of patient characteristics on the clinical efficacy of mongersen (GED-0301), an oral Smad7 antisense oligonucleotide, in active Crohn’s disease. Aliment. Pharmacol. Ther. 2016, 43, 717–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sands, B.E.; Feagan, B.G.; Sandborn, W.J.; Schreiber, S.; Peyrin-Biroulet, L.; Frederic Colombel, J.; Rossiter, G.; Usiskin, K.; Ather, S.; Zhan, X.; et al. Mongersen (GED-0301) for Active Crohn’s Disease: Results of a Phase 3 Study. Am. J. Gastroenterol. 2020, 115, 738–745. [Google Scholar] [CrossRef]
- Bewtra, M.; Lichtenstein, G.R. Mongersen and SMAD-7 Inhibition, Not a Lucky 7 for Patients With IBD: When Trial Design Is as Important as Disease Therapy. Am. J. Gastroenterol. 2020, 115, 687–688. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Sands, B.E.; Rossiter, G.; Li, X.; Usiskin, K.; Zhan, X.; Colombel, J.F. Effects of Mongersen (GED-0301) on Endoscopic and Clinical Outcomes in Patients With Active Crohn’s Disease. Gastroenterology 2018, 154, 61–64.e6. [Google Scholar] [CrossRef]
- Eckstein, F. Phosphorothioates, essential components of therapeutic oligonucleotides. Nucleic Acid Ther. 2014, 24, 374–387. [Google Scholar] [CrossRef]
- Khvorova, A.; Watts, J.K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 2017, 35, 238–248. [Google Scholar] [CrossRef]
- Arrico, L.; Stolfi, C.; Marafini, I.; Monteleone, G.; Demartis, S.; Bellinvia, S.; Viti, F.; McNulty, M.; Cabani, I.; Falezza, A.; et al. Inhomogeneous Diastereomeric Composition of Mongersen Antisense Phosphorothioate Oligonucleotide Preparations and Related Pharmacological Activity Impairment. Nucleic Acid Ther. 2022, 32, 312–320. [Google Scholar] [CrossRef]
- Marafini, I.; Stolfi, C.; Troncone, E.; Lolli, E.; Onali, S.; Paoluzi, O.A.; Fantini, M.C.; Biancone, L.; Calabrese, E.; Di Grazia, A.; et al. A Pharmacological Batch of Mongersen that Downregulates Smad7 is Effective as Induction Therapy in Active Crohn’s Disease: A Phase II, Open-Label Study. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2021, 35, 325–336. [Google Scholar] [CrossRef]
- Duschmale, J.; Hansen, H.F.; Duschmale, M.; Koller, E.; Albaek, N.; Moller, M.R.; Jensen, K.; Koch, T.; Wengel, J.; Bleicher, K. In vitro and in vivo properties of therapeutic oligonucleotides containing non-chiral 3′ and 5′ thiophosphate linkages. Nucleic Acids Res. 2020, 48, 63–74. [Google Scholar] [CrossRef]
- Maresca, C.; Di Maggio, G.; Stolfi, C.; Laudisi, F.; Colella, M.; Pacifico, T.; Di Grazia, A.; Di Fusco, D.; Congiu, D.; Guida, A.M.; et al. Smad7 Sustains Stat3 Expression and Signaling in Colon Cancer Cells. Cancers 2022, 14, 4993. [Google Scholar] [CrossRef] [PubMed]
- Edlund, S.; Lee, S.Y.; Grimsby, S.; Zhang, S.; Aspenstrom, P.; Heldin, C.H.; Landstrom, M. Interaction between Smad7 and beta-catenin: Importance for transforming growth factor beta-induced apoptosis. Mol. Cell. Biol. 2005, 25, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteleone, G.; Stolfi, C. Smad7 Antisense Oligonucleotide in Crohn’s Disease: A Re-Evaluation and Explanation for the Discordant Results of Clinical Trials. Pharmaceutics 2023, 15, 95. https://doi.org/10.3390/pharmaceutics15010095
Monteleone G, Stolfi C. Smad7 Antisense Oligonucleotide in Crohn’s Disease: A Re-Evaluation and Explanation for the Discordant Results of Clinical Trials. Pharmaceutics. 2023; 15(1):95. https://doi.org/10.3390/pharmaceutics15010095
Chicago/Turabian StyleMonteleone, Giovanni, and Carmine Stolfi. 2023. "Smad7 Antisense Oligonucleotide in Crohn’s Disease: A Re-Evaluation and Explanation for the Discordant Results of Clinical Trials" Pharmaceutics 15, no. 1: 95. https://doi.org/10.3390/pharmaceutics15010095