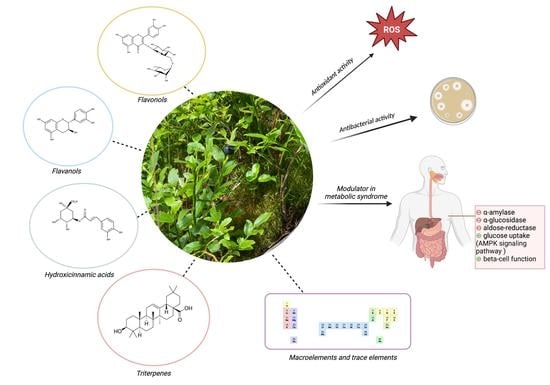
An Updated Systematic Review of Vaccinium myrtillus Leaves: Phytochemistry and Pharmacology
Abstract
:1. Introduction
2. Chemical Composition
2.1. Total Polyphenols
| Concentration mg GAE/g DW | Solvent | Country of Origin | Reference |
|---|---|---|---|
| 500–850 | 80% methanol | Poland | [13] |
| 33–66 | 40% ethanol | Bulgaria | [14] |
| 76.73 | water-glycerin 80:20 | Poland | [15] |
| 54.7–106.9 | 1% aqueous citric acid | Romania | [16] |
| 399.94 | acetone-water 70:30 | Poland | [17] |
| 173–218 | 70% ethanol | Montenegro | [18] |
| 35.9–229.8 | 75% methanol (fresh plant) | Lithuania | [19] |
| 218 | 70% ethanol | Montenegro | [20] |
| 119.17 | water | Bosnia and Herzegovina | [21] |
| 107.79 | ethanol | ||
| 66.76 | ethyl acetate | ||
| 99.3 | 50% ethanol | Ukraine | [22] |
| 68.84 | methanol-ascorbic acid-acetic acid | Poland | [23] |
2.2. Total Flavonoids
2.3. Tannin Content
2.4. Individual Compounds Identified in Vaccinium myrtillus Leaves
| Hydroxycinnamic Acids | Concentration Range mg/g DW | Country of Origin | Reference |
| Chlorogenic acid | 10.04–22.34 | Bulgaria | [14] |
| 3.34–5.94 | Romania | [29] | |
| 11.21–20.82 | Bosnia and Herzegovina | [33] | |
| 45.51–59.7 | Montenegro | [18] | |
| 0.07 | Macedonia | [34] | |
| 1.25–4.14 | Turkey | [32] | |
| 104.7 | Romania | [30] | |
| n.q. | Finland | [35] | |
| n.q. | Poland | [36] | |
| n.q. | Italy | [11] | |
| Neochlorogenic acid | 0.34–3.72 | Montenegro | [18] |
| 2.2 | Macedonia | [34] | |
| n.q. | Italy | [11] | |
| Caffeic acid | 2.67–5.9 | Bulgaria | [14] |
| 1.25–1.95 | Montenegro | [18] | |
| 3.3–28.2 | Turkey | [32] | |
| 3.06 | Romania | [30] | |
| n.q. | Poland | [23] | |
| n.q. | Italy | [11] | |
| p-Coumaric acid | 6.37–12.95 | Bulgaria | [14] |
| 0.32–11.9 | Bosnia and Herzegovina | [33] | |
| 1.26–2.08 | Montenegro | [18] | |
| 8.5–17.6 | Turkey | [32] | |
| n.q. | Italy | [11] | |
| p-Coumaroylquinic acid | 0.07 | Macedonia | [34] |
| n.q. | Italy | [11] | |
| p-Coumaroylhexoside | n.q. | Romania | [16] |
| Ferulic acid | 0.11–0.28 | Montenegro | [18] |
| 3.1–10 | Turkey | [32] | |
| trans-Ferulic acid | 10.95–24.9 | Turkey | [32] |
| Feruloylquinic acid | 47.66–59.65 | Romania | [29] |
| 3,4-Dicaffeoylquinic acid | 0–4.05–5.01 | Romania | [29] |
| 3-O-Caffeoylshikimic acid | n.q. | Romania | [16] |
| n.q. | Italy | [11] | |
| Sinapic acid | 0.18–0.63 | Montenegro | [18] |
| Hydroxybenzoic acids | Concentration range mg/g DW | Country of origin | Reference |
| Gallic acid | 0.54–0.8 | Montenegro | [18] |
| 67.4–352.3 | Turkey | [32] | |
| Syringic acid | 32.5–203 | Turkey | [32] |
| Protocatechuic acid | 176.45 | Bosnia and Herzegovina | [33] |
| 1.4–1.74 | Montenegro | [18] | |
| Vanillic acid | 20.8–1156.8 | Turkey | [32] |
| Other polyphenols | |||
| Pyrogallol | 2.45–3.46 | Montenegro | [18] |
| Resveratrol | 4.6–5.15 | Montenegro | [18] |
| 1.5–8.9 | Turkey | [32] | |
| Flavanols | Concentration range mg/g DW | Country of origin | Reference |
| Gallocatechin | 4.84–15.37 | Romania | [29] |
| n.q. | Hungary | [37] | |
| Epigallocatechin | n.d.–6.56 | Romania | [29] |
| 18.2–197.8 | Turkey | [32] | |
| n.q. | Hungary | [37] | |
| Catechin | 4.79–9.87 | Romania | [29] |
| 7.3–95.6 | Turkey | [32] | |
| 2.32 | Romania | [30] | |
| n.q. | Finland | [35] | |
| 0.21 | Macedonia | [34] | |
| n.q. | Hungary | [37] | |
| Epicatechin | n.d.–9.66 | Romania | [29] |
| 4.38–5.75 | Montenegro | [18] | |
| 10.1–84.1 | Turkey | [32] | |
| 1.95 | Romania | [30] | |
| n.q. | Romania | [16] | |
| n.q. | Finland | [31,35] | |
| n.q. | Hungary | [37] | |
| Procyanidin B2 | 0.31–1.03 | Montenegro | [18] |
| Procyanidin dimer I | 5.55 | Romania | [30] |
| Procyanidin dimer II | 8.7–12.68 | Romania | [29] |
| Procyanidin trimer | 10.09–24.30 | Romania | [29] |
| Procyanidin trimer B | n.q. | Italy | [11] |
| B-type procyanidin dimer I | n.q. | Finland | [31,35] |
| B-type procyanidin dimer II | n.q. | Finland | [35] |
| B-type procyanidin dimer III | n.q. | Finland | [35] |
| Type A/B procyanidin trimer | n.q. | Finland | [35] |
| n.q. | Italy | [11] | |
| A-type procyanidin trimer | n.q. | Estonia | [38] |
| B-type procyanidin trimer | n.q. | Finland | [35] |
| n.q. | Estonia | [38] | |
| Proanthocyanidin tetramer B | n.q. | Italy | [11] |
| Proanthocyanidin pentamer B | n.q. | Italy | [11] |
| Flavonols | Concentration range mg/g DW | Country of origin | Reference |
| Luteolin 5-O-rutinoside | n.q. | Hungary | [37] |
| Myricetin | 49.4–237.6 | Turkey | [32] |
| Quercetin 3-O-rutinoside | 42.24–49.83 | Romania | [29] |
| 4.73–4.94 | Montenegro | [18] | |
| 0.87 | Macedonia | [34] | |
| n.q. | Hungary | [37] | |
| n.q. | Italy | [11] | |
| Quercetin 3-O-glucoside | 1.29–2.37 | Romania | [29] |
| 9.92–16.2 | Montenegro | [18] | |
| 14.5 | Romania | [30] | |
| n.q. | Romania | [16] | |
| n.q. | Italy | [11] | |
| Quercetin 3-O-rhamnoside | n.q. | Finland | [35] |
| 1.65 | Romania | [30] | |
| n.q. | Romania | [16] | |
| 0.11 | Macedonia | [34] | |
| Quercetin-acetyl-rhamnoside | 12.67–18.6 | Romania | [29] |
| 0.98 | Romania | [30] | |
| Quercetin 3-O-arabinoside | 1.39–1.55 | Romania | [29] |
| 0.64 | Romania | [30] | |
| 0.21 | Macedonia | [34] | |
| n.q. | Finland | [35] | |
| n.q. | Italy | [11] | |
| Quercetin-xyloside | 1.3–1.53 | Romania | [29] |
| Quercetin glucosyl-xyloside | 0.7 | Romania | [30] |
| Quercetin-diglucoside | 0.17–1.42 | Romania | [29] |
| Quercetin 3-O-galactoside | 32.16–44.43 | Bosnia and Herzegovina | [33] |
| 2.38–2.55 | Montenegro | [18] | |
| 2.45 | Macedonia | [34] | |
| n.q. | Finland | [31,35] | |
| n.q. | Romania | [16] | |
| n.q. | Hungary | [37] | |
| n.q. | Poland | [23] | |
| Quercetin 3-O-glucuronide | n.q. | Finland | [31,35] |
| n.q. | Hungary | [37] | |
| n.q. | Italy | [11] | |
| Quercetin-hexuronide | n.q. | Romania | [16] |
| Quercetin 3-sambubioside | n.q. | Hungary | [37] |
| Quercetin | 1.16–3.69 | Romania | [29] |
| 24.75–85.64 | Bosnia and Herzegovina | [33] | |
| 1.16–7.27 | Montenegro | [18] | |
| 2.1–11.4 | Turkey | [32] | |
| n.q. | Hungary | [37] | |
| Kaempferol 3-O-glucoside | 1.38–1.6 | Montenegro | [18] |
| Kaempferol 3-O-glucuronide | n.q. | Finland | [35,39] |
| n.q. | Italy | [11] | |
| Kaempferol-rhamnoside | n.q. | Finland | [35] |
| Kaempferol-hexuronide | n.q. | Romania | [16] |
| Kaempferol | 3.45 | Bosnia and Herzegovina | [33] |
| 0.03–0.26 | Montenegro | [18] | |
| 1.6–3.4 | Turkey | [32] | |
| Taxifolin | n.q. | Hungary | [37] |
| Flavanolignans | |||
| Cinchonain I | n.q. | Italy | [11] |
| Cinchonain II | n.q. | Italy | [11] |
| Anthocyanins | Concentration range µg/g DW | Country of origin | Reference |
| Cyanidin-glucoside | n.d.–0.29 | Romania | [29] |
| n.d.–1.06 | Turkey | [32] | |
| Cyanidin-arabinoside | n.d.–0.3 | Romania | [29] |
| Cyanidin-acetyl-glucoside | n.d.–0.33 | Romania | [29] |
| Malvidin 3-O-glucoside | n.d.–1.2 | Turkey | [32] |
| Compound | Concentration Range µg/g DW | Country of Origin | Reference |
|---|---|---|---|
| Triterpenes | |||
| Oleanolic acid | 505–655 | Bulgaria | [43] |
| 853.2 | Finland | [44] | |
| 873.6 | Poland | [44] | |
| Ursolic acid | 377–815 | Bulgaria | [43] |
| 747.7 | Finland | [44] | |
| 776.2 | Poland | [44] | |
| Lupeol | 20–55 | Bulgaria | [43] |
| 24.6 | Finland | [44] | |
| 63.4 | Poland | [44] | |
| α-Amyrin | 102–568 | Bulgaria | [43] |
| 711.9 | Finland | [44] | |
| 631.4 | Poland | [44] | |
| α-Amyrenone | 11.7 | Finland | [44] |
| 22.2 | Poland | [44] | |
| β-Amyrin | 919.1 | Finland | [44] |
| 987.2 | Poland | [44] | |
| β-Amyrenone | 15.4 | Finland | [44] |
| 34.7 | Poland | [44] | |
| 22.8 | Poland | [44] | |
| Hydroxyoleanolic acid | 50.1 | Finland | [44] |
| 164.7 | Poland | [44] | |
| Hydroxyursolic acid | 33.9 | Finland | [44] |
| 115.8 | Poland | [44] | |
| Phytosterols | |||
| Cycloartanol | 20.3 | Finland | [44] |
| Campesterol | 11.1 | Finland | [44] |
| 16.7 | Poland | [44] | |
| Sitostanol | 6.9 | Finland | [44] |
| 8.2 | Poland | [44] | |
| Sitosterol | 610.9 | Finland | [44] |
| 671.4 | Poland | [44] | |
| Stigmastadienone | 38.3 | Finland | [44] |
| 20.8 | Poland | [44] | |
| Stigmasterol | 5.4 | Finland | [44] |
| 5.2 | Poland | [44] | |
2.5. Macroelements and Trace Elements
3. Pharmacokinetics of Polyphenols
- the importance of the food matrix in the case of the administration of natural extracts, as well as the presence of dimer or trimer forms for catechins and proanthocyanidins (in general, analytical methods only quantify the monomers present in the plasma in bioavailability studies) [57];
- the metabolic processes of deglycosylation that take place at the level of the enterocyte, which lead to the formation of aglycones: intracellular capture by SGLT1 (sodium-glucose co-transporter 1), followed by hydrolysis under the action of CBG (cytosolic beta-glucosidase), respectively, LPH (lactase-phlorizine hydrolase);
- biotransformation in the intestinal wall (via the cytochrome P450 isoform CYP3A4) and in the liver (many CYP450 isoenzymes, but mainly CYP3A4), which gives oxidized metabolites of the initial aglycones; subsequently, the latter can undergo phase II reactions and conjugation with endogenous compounds: glucuronides are obtained (under the action of UGT-uridine-5’-diphosphate glucuronyl transferase), sulfates via SULT (sulfotransferases, with PAPS as co-factor—phospho-adenosyl-phosphosulfate), respectively, compounds methylated via COMT (catechol-O-methyltransferase), as shown in Figure 3 [58];
- glucuronide and sulfate metabolites may undergo enterohepatic circulation due to biliary elimination and reabsorption in the duodenum; some of the compounds already modified can undergo enzymatic hydrolysis and reabsorption in the portal vein;
- the unabsorbed polyphenolic fraction reaches the colon, where it undergoes complex enzymatic processes under the action of saprophytic flora; the newly formed compounds can be absorbed and will be available in the systemic circulation [59].
4. Pharmacological Activity
4.1. Antioxidant Capacity
4.2. Antimicrobial Activity
4.3. Metabolic Modulation
4.4. Dermatological Effects
5. Safety Evaluation
6. Previous Published Reviews
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Evans, W. Trease and Evans’ Pharmacognosy, 16th ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019; Volume 1. [Google Scholar]
- EMA Herbal Medicinal Products. Available online: https://www.ema.europa.eu/en/human-regulatory/herbal-medicinal-products (accessed on 7 December 2022).
- Ősz, B.-E.; Jîtcă, G.; Ștefănescu, R.-E.; Pușcaș, A.; Tero-Vescan, A.; Vari, C.-E. Caffeine and Its Antioxidant Properties—It Is All about Dose and Source. Int. J. Mol. Sci. 2022, 23, 13074. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Ștefănescu, B.E.; Szabo, K.; Mocan, A.; Crişan, G. Phenolic Compounds from Five Ericaceae Species Leaves and Their Related Bioavailability and Health Benefits. Molecules 2019, 24, 2046. [Google Scholar] [CrossRef] [Green Version]
- Savych, A.; Marchyshyn, S.; Mosula, L.; Bilyk, O.; Humeniuk, I.; Davidenko, A. Analysis of Amino Acids Content in the Plant Components of the Antidiabetic Herbal Mixture by GC-MS. Pharmacia 2022, 69, 69–76. [Google Scholar] [CrossRef]
- Savych, A.; Basaraba, R.; Muzyka, N.; Ilashchuk, P. Analysis of Fatty Acid Composition Content in the Plant Components of Antidiabetic Herbal Mixture by GC-MS. Pharmacia 2021, 68, 433–439. [Google Scholar] [CrossRef]
- Madić, V.; Petrović, A.; Jušković, M.; Jugović, D.; Djordjević, L.; Stojanović, G.; Vasiljević, P. Polyherbal Mixture Ameliorates Hyperglycemia, Hyperlipidemia and Histopathological Changes of Pancreas, Kidney and Liver in a Rat Model of Type 1 Diabetes. J. Ethnopharmacol. 2021, 265, 113210. [Google Scholar] [CrossRef]
- Madić, V.; Stojanović-Radić, Z.; Jušković, M.; Jugović, D.; Žabar Popović, A.; Vasiljević, P. Genotoxic and Antigenotoxic Potential of Herbal Mixture and Five Medicinal Plants Used in Ethnopharmacology. S. Afr. J. Bot. 2019, 125, 290–297. [Google Scholar] [CrossRef]
- Ieri, F.; Martini, S.; Innocenti, M.; Mulinacci, N. Phenolic Distribution in Liquid Preparations of Vaccinium myrtillus L. and Vaccinium vitis idaea L.: Analysis of Liquid Preparation of Bilberry and Lingonberry. Phytochem. Anal. 2013, 24, 467–475. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Kandziora-Ciupa, M.; Dabioch, M.; Nadgórska-Socha, A. Evaluating the Accumulation of Antioxidant and Macro- and Trace Elements in Vaccinium myrtillus L. Biol. Trace Elem. Res. 2021, 200, 4175–4185. [Google Scholar] [CrossRef]
- Vrancheva, R.; Ivanov, I.; Badjakov, I.; Dincheva, I.; Georgiev, V.; Pavlov, A. Intrapopulation Variation of Polyphenolic Compounds with Antioxidant Potential in Bulgarian Bilberry (Vaccinium myrtillus L.). C. R. Acad. Bulg. Sci. 2021, 74, 1781–1788. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Assessment of Cytotoxicity and Antioxidant Properties of Berry Leaves as By-Products with Potential Application in Cosmetic and Pharmaceutical Products. Sci. Rep. 2021, 11, 3240. [Google Scholar] [CrossRef] [PubMed]
- Bujor, O.-C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal Variations of the Phenolic Constituents in Bilberry (Vaccinium myrtillus L.) Leaves, Stems and Fruits, and Their Antioxidant Activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, B.; Paszkiewicz, M.; Podsędek, A.; Redzynia, M.; Różalska, B. Vaccinium myrtillus Leaves and Frangula Alnus Bark Derived Extracts as Potential Antistaphylococcal Agents. Acta Biochim. Pol. 2014, 61, 163–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasanac-Vukanovic, S.; Mutic, J.; Stankovic, D.; Arsic, I.; Blagojevic, N.; Vukasinovic-Pesic, V.; Tadic, V. Wild Bilberry (Vaccinium myrtillus L., Ericaceae) from Montenegro as a Source of Antioxidants for Use in the Production of Nutraceuticals. Molecules 2018, 23, 1864. [Google Scholar] [CrossRef] [Green Version]
- Kaškonienė, V.; Bimbiraitė-Survilienė, K.; Kaškonas, P.; Tiso, N.; Česonienė, L.; Daubaras, R.; Maruška, A.S. Changes in the Biochemical Compounds of Vaccinium myrtillus, Vaccinium vitis-idaea, and Forest Litter Collected from Various Forest Types. Turk. J. Agric. For. 2020, 44, 557–566. [Google Scholar] [CrossRef]
- Tadić, V.M.; Nešić, I.; Martinović, M.; Rój, E.; Brašanac-Vukanović, S.; Maksimović, S.; Žugić, A. Old Plant, New Possibilities: Wild Bilberry (Vaccinium myrtillus L., Ericaceae) in Topical Skin Preparation. Antioxidants 2021, 10, 465. [Google Scholar] [CrossRef]
- Dragana, M.V.; Miroslav, R.P.; Branka, B.R.G.; Olgica, D.S.; Sava, M.V.; Ljiljana, R.Č. Antibacterial and Antioxidant Activities of Bilberry (Vaccinium myrtillus L.) In Vitro. Afr. J. Microbiol. Res. 2013, 7, 5130–5136. [Google Scholar] [CrossRef] [Green Version]
- Zagayko, A.L.; Kolisnyk, T.Y.; Chumak, O.I.; Ruban, O.A.; Koshovyi, O.M. Evaluation of Anti-Obesity and Lipid-Lowering Properties of Vaccinium myrtillus Leaves Powder Extract in a Hamster Model. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 697–703. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdyło, A. Comparison of Phenolic Compounds and Antioxidant Potential between Selected Edible Fruits and Their Leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Hăncianu, M.; Gîrd, C.E. Farmacognozie. Produse Vegetale cu Substanțe Bioactive; Stănescu, U., Ed.; Polirom: Bucharest, Romania, 2020. [Google Scholar]
- Tao, W.; Zhang, Y.; Shen, X.; Cao, Y.; Shi, J.; Ye, X.; Chen, S. Rethinking the Mechanism of the Health Benefits of Proanthocyanidins: Absorption, Metabolism, and Interaction with Gut Microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 971–985. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of Dietary Polyphenols: The Role of Metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Valiñas, M.A.; Lanteri, M.L.; ten Have, A.; Andreu, A.B. Chlorogenic Acid, Anthocyanin and Flavan-3-Ol Biosynthesis in Flesh and Skin of Andean Potato Tubers (Solanum tuberosum Subsp. Andigena). Food Chem. 2017, 229, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical Composition and Biological Activities of the Nord-West Romanian Wild Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.) Leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef]
- Ștefănescu, B.E.; Nemes, S.-A.; Teleky, B.-E.; Călinoiu, L.F.; Mitrea, L.; Martău, G.A.; Szabo, K.; Mihai, M.; Vodnar, D.C.; Crișan, G. Microencapsulation and Bioaccessibility of Phenolic Compounds of Vaccinium Leaf Extracts. Antioxidants 2022, 11, 674. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Alanne, A.-L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic Compounds Extracted by Acidic Aqueous Ethanol from Berries and Leaves of Different Berry Plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef]
- Değirmencioğlu, N.; Gürbüz, O.; Karatepe, G.E.; Irkin, R. Influence of Hot Air Drying on Phenolic Compounds and Antioxidant Capacity of Blueberry (Vaccinium myrtillus) Fruit and Leaf. J. Appl. Bot. Food Qual. 2017, 90, 15–125. [Google Scholar] [CrossRef]
- Bljajić, K.; Petlevski, R.; Vujić, L.; Čačić, A.; Šoštarić, N.; Jablan, J.; Saraiva de Carvalho, I.; Zovko Končić, M. Chemical Composition, Antioxidant and α-Glucosidase-Inhibiting Activities of the Aqueous and Hydroethanolic Extracts of Vaccinium myrtillus Leaves. Molecules 2017, 22, 703. [Google Scholar] [CrossRef]
- Stefkov, G.; Hristovski, S.; Petreska Stanoeva, J.; Stefova, M.; Melovski, L.; Kulevanova, S. Resource Assessment and Economic Potential of Bilberries (Vaccinium myrtillus and Vaccinium uliginosum) on Osogovo Mtn., R. Macedonia. Ind. Crops Prod. 2014, 61, 145–150. [Google Scholar] [CrossRef]
- Liu, P.; Lindstedt, A.; Markkinen, N.; Sinkkonen, J.; Suomela, J.-P.; Yang, B. Characterization of Metabolite Profiles of Leaves of Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.). J. Agric. Food Chem. 2014, 62, 12015–12026. [Google Scholar] [CrossRef]
- Cyboran, S.; Oszmiański, J.; Kleszczyńska, H. Modification of the Lipid Phase of Biological and Model Membranes by Bilberry Leaf Extract. Food Biophys. 2013, 8, 321–333. [Google Scholar] [CrossRef]
- Takács, I.; Szekeres, A.; Takács, Á.; Rakk, D.; Mézes, M.; Polyák, Á.; Lakatos, L.; Gyémánt, G.; Csupor, D.; Kovács, K.J.; et al. Wild Strawberry, Blackberry, and Blueberry Leaf Extracts Alleviate Starch-Induced Hyperglycemia in Prediabetic and Diabetic Mice. Planta Med. 2020, 86, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Toomik, P.; Püssa, T.; Raal, A. Variability of Procyanidin Type A- and -B Trimers Content in Aerial Parts of Some Vaccinium Species and Cultivars. Nat. Prod. Commun. 2014, 9, 815–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martz, F.; Jaakola, L.; Julkunen-Tiitto, R.; Stark, S. Phenolic Composition and Antioxidant Capacity of Bilberry (Vaccinium myrtillus) Leaves in Northern Europe Following Foliar Development and Along Environmental Gradients. J. Chem. Ecol. 2010, 36, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Bujor, A.; Miron, A.; Aprotosoaie, A.C.; Skalicka-Woźniak, K.; Trifan, A. Preparative Separation and Bioactivity of Oligomeric Proanthocyanidins. Phytochem. Rev. 2020, 19, 1093–1140. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Matkowski, A.; Hadzik, J.; Dobrowolska-Czopor, B.; Olchowy, C.; Dominiak, M.; Kubasiewicz-Ross, P. Proanthocyanidins and Flavan-3-Ols in the Prevention and Treatment of Periodontitis—Antibacterial Effects. Nutrients 2021, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Hohtola, A.; Jaakola, L.; Määttä-Riihinen, K.; Kärenlampi, S. Activation of Flavonoid Biosynthesis by Solar Radiation in Bilberry (Vaccinium myrtillus L.) Leaves. Planta 2004, 218, 721–728. [Google Scholar] [CrossRef]
- Vrancheva, R.; Ivanov, I.; Dincheva, I.; Badjakov, I.; Pavlov, A. Triterpenoids and Other Non-Polar Compounds in Leaves of Wild and Cultivated Vaccinium Species. Plants 2021, 10, 94. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Huttunen, S. Triterpenoid Content of Berries and Leaves of Bilberry Vaccinium myrtillus from Finland and Poland. J. Agric. Food Chem. 2012, 60, 11839–11849. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Bangladesh Journal of Botany. Chemical Composition and Biological Activities of the Essential Oil from the Leaves of Vaccinium myrtillus L. Available online: https://www.banglajol.info/index.php/BJB/article/view/49098 (accessed on 9 November 2022).
- Morkunas, I.; Woźniak, A.; Mai, V.C.; Rucińska-Sobkowiak, R.; Jeandet, P. The Role of Heavy Metals in Plant Response to Biotic Stress. Molecules 2018, 23, 2320. [Google Scholar] [CrossRef] [Green Version]
- Kandziora-Ciupa, M.; Gospodarek, J.; Nadgórska-Socha, A. Pollution and Ecological Risk Assessment of Heavy Metals in Forest Soils with Changes in the Leaf Traits and Membrane Integrity of Vaccinium myrtillus L. Eur. J. For. Res. 2022, 141, 409–419. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Lead in Food, Foodwares, and Dietary Supplements; FDA: Silver Spring, MD, USA, 2020. [Google Scholar]
- Amelia, T.; Bianca-Eugenia, O.; Amalia, M. Substantele Minerale, Factori Esentiali in Nutritie. O Abordare Din Perspectiva Bio-Chimica Si Farmacologica; University Press: Targu Mures, Romania, 2020; ISBN 978-973-169-642-3. [Google Scholar]
- Nordløkken, M.; Berg, T.; Flaten, T.P.; Steinnes, E. Essential and Non-Essential Elements in Natural Vegetation in Southern Norway: Contribution from Different Sources. Sci. Total Environ. 2015, 502, 391–399. [Google Scholar] [CrossRef]
- Eeva, T.; Holmström, H.; Espín, S.; Sánchez-Virosta, P.; Klemola, T. Leaves, Berries and Herbivorous Larvae of Bilberry Vaccinium myrtillus as Sources of Metals in Food Chains at a Cu-Ni Smelter Site. Chemosphere 2018, 210, 859–866. [Google Scholar] [CrossRef]
- Kandziora-Ciupa, M.; Ciepał, R.; Nadgórska-Socha, A.; Barczyk, G. A Comparative Study of Heavy Metal Accumulation and Antioxidant Responses in Vaccinium myrtillus L. Leaves in Polluted and Non-Polluted Areas. Environ. Sci. Pollut. Res. 2013, 20, 4920–4932. [Google Scholar] [CrossRef] [Green Version]
- Miljković, V.M.; Nikolić, G.S.; Zvezdanović, J.; Mihajlov-Krstev, T.; Arsić, B.B.; Miljković, M.N. Phenolic Profile, Mineral Content and Antibacterial Activity of the Methanol Extract of Vaccinium myrtillus L. Not. Bot. Hortic. Agrobot. 2018, 46, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Khodavirdipour, A.; Haddadi, F.; Keshavarzi, S. Chromium Supplementation; Negotiation with Diabetes Mellitus, Hyperlipidemia and Depression. J. Diabetes Metab. Disord. 2020, 19, 585–595. [Google Scholar] [CrossRef]
- Kooshki, F.; Tutunchi, H.; Vajdi, M.; Karimi, A.; Niazkar, H.R.; Shoorei, H.; Pourghassem Gargari, B. A Comprehensive Insight into the Effect of Chromium Supplementation on Oxidative Stress Indices in Diabetes Mellitus: A Systematic Review. Clin. Exp. Pharmacol. Physiol. 2021, 48, 291–309. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordoñez, M.; Llorach, R.; Farran-Codina, A.; Barupal, D.K.; Neveu, V.; Manach, C.; Andres-Lacueva, C.; Scalbert, A. Systematic Analysis of the Polyphenol Metabolome Using the Phenol-Explorer Database. Mol. Nutr. Food Res. 2016, 60, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid Metabolism: The Interaction of Metabolites and Gut Microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-Antioxidant Activity Relationship of Methoxy, Phenolic Hydroxyl, and Carboxylic Acid Groups of Phenolic Acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Teng, H.; Xie, Z.; Cao, H.; Cheang, W.S.; Skalicka-Woniak, K.; Georgiev, M.I.; Xiao, J. Modifications of Dietary Flavonoids towards Improved Bioactivity: An Update on Structure–Activity Relationship. Crit. Rev. Food Sci. Nutr. 2018, 58, 513–527. [Google Scholar] [CrossRef]
- Jîtcă, G.; Ősz, B.E.; Tero-Vescan, A.; Miklos, A.P.; Rusz, C.-M.; Bătrînu, M.-G.; Vari, C.E. Positive Aspects of Oxidative Stress at Different Levels of the Human Body: A Review. Antioxidants 2022, 11, 572. [Google Scholar] [CrossRef]
- Olszowy, M. What Is Responsible for Antioxidant Properties of Polyphenolic Compounds from Plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Puganen, A.; Alakomi, H.-L.; Uusitupa, A.; Saarela, M.; Yang, B. Antioxidative and Antibacterial Activities of Aqueous Ethanol Extracts of Berries, Leaves, and Branches of Berry Plants. Food Res. Int. 2018, 106, 291–303. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef] [Green Version]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]
- Rotaru, L.T. Correlation of the Theoretical Study with the Experimental Determination of the Antibacterial Effect of Vaccinium myrtillus Folium (VM-f) Plant Extract. Rev. Chim. 2019, 70, 990–992. [Google Scholar] [CrossRef]
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, A.; Sbar, E. Aflatoxin Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Vamvakas, S.-S.; Chroni, M.; Genneos, F.; Gizeli, S. Vaccinium myrtillus L. Dry Leaf Aqueous Extracts Suppress Aflatoxins Biosynthesis by Aspergillus Flavus. Food Biosci. 2021, 39, 100790. [Google Scholar] [CrossRef]
- Gregg, E.W.; Sattar, N.; Ali, M.K. The Changing Face of Diabetes Complications. Lancet Diabetes Endocrinol. 2016, 4, 537–547. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary Polyphenols as Potential Nutraceuticals in Management of Diabetes: A Review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A Review of Alpha-Glucosidase Inhibitors from Plants as Potential Candidates for the Treatment of Type-2 Diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, D.N.A.W.; Uluwaduge, D.I.; Siriwardhene, M.A. Mechanisms of Action of Sri Lankan Herbal Medicines Used in the Treatment of Diabetes: A Review. J. Integr. Med. 2020, 18, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Magalhães, P.O.; Silveira, D. α-Amylase Inhibitors: A Review of Raw Material and Isolated Compounds from Plant Source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraga-Corral, M.; Otero, P.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Jarboui, A.; Nuñez-Estevez, B.; Simal-Gandara, J.; Prieto, M.A. By-Products of Agri-Food Industry as Tannin-Rich Sources: A Review of Tannins’ Biological Activities and Their Potential for Valorization. Foods 2021, 10, 137. [Google Scholar] [CrossRef]
- Márquez Campos, E.; Jakobs, L.; Simon, M.-C. Antidiabetic Effects of Flavan-3-Ols and Their Microbial Metabolites. Nutrients 2020, 12, 1592. [Google Scholar] [CrossRef]
- Sidorova, Y.; Shipelin, V.; Mazo, V.; Zorin, S.; Petrov, N.; Kochetkova, A. Hypoglycemic and Hypolipidemic Effect of Vaccinium myrtillus L. Leaf and Phaseolus vulgaris L. Seed Coat Extracts in Diabetic Rats. Nutrition 2017, 41, 107–112. [Google Scholar] [CrossRef]
- Ştefănescu (Braic), R.; Vari, C.; Imre, S.; Huţanu, A.; Fogarasi, E.; Todea, T.; Groşan, A.; Eşianu, S.; Laczkó-Zöld, E.; Dogaru, M. Vaccinium Extracts as Modulators in Experimental Type 1 Diabetes. J. Med. Food 2018, 21, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, Y.; Li, X.; Zhu, L.; Wang, X.; Li, L.; Sun, H.; Han, X.; Li, J. Myricetin Relieves the Symptoms of Type 2 Diabetes Mice and Regulates Intestinal Microflora. Biomed. Pharmacother. 2022, 153, 113530. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, R. Quercetin for Managing Type 2 Diabetes and Its Complications, an Insight into Multitarget Therapy. Biomed. Pharmacother. 2022, 146, 112560. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.-J.; Li, Y.; Cao, Q.-H.; Wu, H.-X.; Tang, X.-Y.; Gao, X.-H.; Yu, J.-Q.; Chen, Z.; Yang, Y. In Vitro and in Vivo Evidence That Quercetin Protects against Diabetes and Its Complications: A Systematic Review of the Literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-Diabetic and Anti-Lipidemic Effects of Chlorogenic Acid Are Mediated by Ampk Activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef]
- Alim, Z.; Kilinç, N.; Şengül, B.; Beydemir, Ş. Inhibition Behaviours of Some Phenolic Acids on Rat Kidney Aldose Reductase Enzyme: An in Vitro Study. J. Enzyme Inhib. Med. Chem. 2017, 32, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Ștefanescu, R.; Fülöp, E.; Demian, L.; Vari, C.; Ősz, B.-E.; Groșan, A.; Laczko-Zöld, E.; Chibelean, B. Efficacy of Natural Polyphenolic Compounds from Bilberry and Blueberry on The Metabolic Alterations Induced By Streptozotocin In Rats. Farmacia 2022, 70, 658–664. [Google Scholar]
- Dostalek, M.; Akhlaghi, F.; Puzanovova, M. Effect of Diabetes Mellitus on Pharmacokinetic and Pharmacodynamic Properties of Drugs. Clin. Pharmacokinet. 2012, 51, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, N.R. Strategies for Preclinical Pharmacokinetic Investigation in Streptozotocin-Induced Diabetes Mellitus (DMIS) and Alloxan-Induced Diabetes Mellitus (DMIA) Rat Models: Case Studies and Perspectives. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stoica, M.C.; Vari, C.E.; Imre, S.; Vancea, S.; Dogaru, M.T.; Cara, E.; Tar, D. Correlations Between the Stages of Kidney Disease and the Pharmacokinetic Parameters of Orally Administered Ciprofloxacin at Patients with Chronic Kidney Disease. Farmacia 2015, 63, 6. [Google Scholar]
- Popova, A.; Mihaylova, D. Antinutrients in Plant-Based Foods: A Review. Open Biotechnol. J. 2019, 13, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Chehri, A.; Yarani, R.; Yousefi, Z.; Shakouri, S.K.; Ostadrahimi, A.; Mobasseri, M.; Araj-Khodaei, M. Phytochemical and Pharmacological Anti-Diabetic Properties of Bilberries (Vaccinium myrtillus), Recommendations for Future Studies. Prim. Care Diabetes 2022, 16, 27–33. [Google Scholar] [CrossRef]
- Tundis, R.; Tenuta, M.C.; Loizzo, M.R.; Bonesi, M.; Finetti, F.; Trabalzini, L.; Deguin, B. Vaccinium Species (Ericaceae): From Chemical Composition to Bio-Functional Activities. Appl. Sci. 2021, 11, 5655. [Google Scholar] [CrossRef]




| Concentration mg QE/g DW | Country of Origin | Reference |
|---|---|---|
| 12–35 | Bulgaria | [14] |
| 16.36 | Poland | [15] |
| 25.92 | Poland | [17] |
| 14.6–23.8 | Montenegro | [18] |
| 8–40 | Lithuania | [19] |
| 21.5 | Montenegro | [20] |
| 19.3 | Ukraine | [22] |
| Concentration % (w/w) | Country of Origin | Reference |
|---|---|---|
| 1.54 (hydrolysable, HPLC) | Poland | [17] |
| 6.49–9.17 | Montenegro | [18] |
| 9.17 | Montenegro | [20] |
| Concentration mg/g | Country of Origin | Reference |
|---|---|---|
| 2–5 | Bulgaria | [14] |
| 8.03 | Poland | [15] |
| 25.45 | Poland | [17] |
| 24.75 | Poland | [23] |
| Element | Concentration | Location | Reference |
| Al | 7.45–186.2 µg/g extr | Montenegro | [18] |
| 0.09 mg/g | Norway | [51] | |
| 98–175 mg/kg | Poland | [13] | |
| As | 0.059–0.297 µg/g extr | Montenegro | [18] |
| 1.02 µg/g | Finland (polluted area) | [52] | |
| 0.02 µg/g | Norway | [51] | |
| B | 31 µg/g | Norway | [51] |
| Ba | 5.36–69.6 µg/g extr | Montenegro | [18] |
| 0.028 mg/g | Norway | [51] | |
| Cd | 0.016–0.112 µg/g extr | Montenegro | [18] |
| 0.09–6.26 µg/g | Poland | [53] | |
| 0.12 µg/g | Finland (polluted area) | [52] | |
| 0.02 µg/g | Norway | [51] | |
| 0.34–9.81 mg/kg | Poland | [13] | |
| 0.81–4.43 mg/g | Poland | [48] | |
| Co | 0.055 µg/g extr | Montenegro | [18] |
| 0.3 µg/g | Finland (polluted area) | [52] | |
| 0.02 µg/g | Norway | [51] | |
| Ni | 2.18–4.4 µg/g extr | Montenegro | [18] |
| 12.9 µg/g | Finland (polluted area) | [52] | |
| 0.4 µg/g | Norway | [51] | |
| 1.93–2.17 mg/g | Poland | [48] | |
| Pb | 0.47–0.63 µg/g extract | Montenegro | [18] |
| 0.0036–0.157 mg/g | Poland | [53] | |
| 0.00085 mg/g | Finland (polluted area) | [52] | |
| 0.0003 mg/g | Norway | [51] | |
| 3.26–106 mg/g | Poland | [48] | |
| 4.55–232.1 mg/kg | Poland | [13] | |
| Sr | 6–14.7 µg/g extr | Montenegro | [18] |
| 0.007 mg/g | Norway | [51] | |
| Micronutrient | Concentration | Location | Reference |
| Cr | 0.123–1.11 µg/g extr | Montenegro | [18] |
| Cu | 2.99–33.31 µg/g extr | Montenegro | [18] |
| 20.6 µg/g | Finland (polluted area) | [52] | |
| 7.3 µg/g | Norway | [51] | |
| 6.29–8.32 mg/g | Poland | [48] | |
| 2.17–9.07 mg/kg | Poland | [13] | |
| Fe | 17.4–25.8 µg/g | Montenegro | [18] |
| 0.045 mg/g | Norway | [51] | |
| 59.1–379.4 mg/g | Poland | [48] | |
| 9.36–410 mg/g | Poland | [53] | |
| 59.6–108.6 mg/kg | Poland | [13] | |
| Mn | 251.4–1210 µg/g extr | Montenegro | [18] |
| 38.8–1139 mg/g | Poland | [53] | |
| 39.9–1124.8 mg/kg | Poland | [13] | |
| 414 µg/g | Finland (polluted area) | [52] | |
| 0.98 mg/g | Norway | [51] | |
| 59.3–416.9 mg/g | Poland | [48] | |
| Mo | 0.26 µg/g | Finland (polluted area) | [52] |
| 0.05 µg/g | Norway | [51] | |
| Se | 0.19 µg/g | Finland (polluted area) | [52] |
| 0.1 µg/g | Norway | [51] | |
| Zn | 20.5–31.5 µg/g | Montenegro | [18] |
| 11.2–207 mg/g | Poland | [53] | |
| 15.6 µg/g | Finland (polluted area) | [52] | |
| 21.7–99.1 mg/g | Poland | [48] | |
| 0.021 mg/g | Norway | [51] | |
| 12.2–332.3 mg/kg | Poland | [13] | |
| Macronutrient | Concentration mg/g | Location | Reference |
| K | 8.2–17.2 | Montenegro | [18] |
| 13.3 | Serbia | [54] | |
| 7.3 | Norway | [51] | |
| 3.3–4.99 | Poland | [13] | |
| Na | 0.138–1.262 | Montenegro | [18] |
| 0.09 | Norway | [51] | |
| 1.7–2.2 | Poland | [13] | |
| Mg | 1.5–3.8 | Montenegro | [18] |
| 0.41 | Serbia | [54] | |
| 1.4 | Norway | [51] | |
| 0.5–1.3 | Poland | [13] | |
| P | 1.1 | Norway | [51] |
| 3.13–3.3 | Poland | [13] | |
| Ca | 1.2–5.9 | Montenegro | [18] |
| 0.007 | Finland (polluted area) | [52] | |
| 2.36 | Serbia | [54] | |
| 5.6 | Norway | [51] | |
| S | 1.7 | Norway | [51] |
| 2.94–4 | Poland | [13] |
| Strain | MIC mg/mL | Reference |
|---|---|---|
| Gram positive | ||
| Bacillus cereus | + | [65] |
| Staphylococcus aureus ATCC 49444 | 0.06–0.12 | [29] |
| ATCC 29213 | 1.5 | [17] |
| clinical strain | 0.75 | |
| + | [65] | |
| ATCC 25923 | + | [68] |
| wound swabs | 63 | [54] |
| ATCC 6538 | 63 | |
| Staphylococcus epidermidis | [54] | |
| wound swabs | 15.75 | |
| ATCC 12228 | 15.75 | |
| Streptococcus pyogenes | ||
| ATCC 19615 | 31.5 | [54] |
| wound swabs | 31.5 | |
| Enterococcus faecalis ATCC 29212 | 0.24–0.48 | [29] |
| clinical isolate | 20–40 | [21] |
| Propionibacterium acnae | ||
| ATCC 11827 | 126 | [54] |
| R. equi. ATCC 6939 | 0.12 | [29] |
| Listeria monocytogenes | + | [65] |
| Gram negative | ||
| E. coli ATCC 25922 | 0.48–0.96 | [29] |
| + | [68] | |
| clinical isolate | 40 | [21] |
| + | [65] | |
| K. pneumoniae DSMZ 2026 | 0.24–0.48 | [29] |
| ATCC 10031 | 126 | [54] |
| wound swabs | 126 | |
| ATCC 700603 | + | [68] |
| P. aeruginosa ATCC 27853 | 0.48 | [29] |
| + | [68] | |
| ATCC 9027 | 31.5 | [54] |
| wound swabs | 31.5 | |
| Salmonella enterica | + | [65] |
| Acinetobacter boumanii | ||
| ATCC 196060 | 63 | [54] |
| Acinetobacter sp. | ||
| wound swabs | 252 | [54] |
| Proteus mirabilis | ||
| ATCC 12453 | 31.5 | [54] |
| wound swabs | 63 | |
| Proteus vulgaris clinical isolate | 20 | [21] |
| ATCC 6380 | + | [68] |
| Fungi | ||
| Candida albicans ATCC 10231 | 250 | [29] |
| Candida zeylanoides ATCC 20367 | 62.5–125 | [29] |
| Candida parapsilosis ATCC 22019 | 62.5 | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ștefănescu, R.; Laczkó-Zöld, E.; Ősz, B.-E.; Vari, C.-E. An Updated Systematic Review of Vaccinium myrtillus Leaves: Phytochemistry and Pharmacology. Pharmaceutics 2023, 15, 16. https://doi.org/10.3390/pharmaceutics15010016
Ștefănescu R, Laczkó-Zöld E, Ősz B-E, Vari C-E. An Updated Systematic Review of Vaccinium myrtillus Leaves: Phytochemistry and Pharmacology. Pharmaceutics. 2023; 15(1):16. https://doi.org/10.3390/pharmaceutics15010016
Chicago/Turabian StyleȘtefănescu, Ruxandra, Eszter Laczkó-Zöld, Bianca-Eugenia Ősz, and Camil-Eugen Vari. 2023. "An Updated Systematic Review of Vaccinium myrtillus Leaves: Phytochemistry and Pharmacology" Pharmaceutics 15, no. 1: 16. https://doi.org/10.3390/pharmaceutics15010016