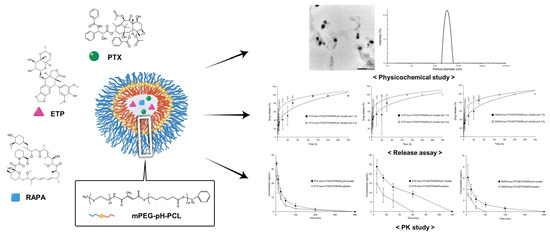
Evaluation of pH-Sensitive Polymeric Micelles Using Citraconic Amide Bonds for the Co-Delivery of Paclitaxel, Etoposide, and Rapamycin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cell Line and Cell Culture
2.3. CI Analysis
2.4. Preparation of PTX/ETP/RAPA-Loaded Polymeric Micelles
2.5. Physicochemical Characterization of Micelles
2.6. Transmission Electron Microscopy (TEM) Observation
2.7. In Vitro pH-Sensitivity Assessment of Micelles
2.8. In Vitro Cytotoxicity Assay
2.9. In Vitro Clonogenic Assay
2.10. In Vitro Drug Release Assay
2.11. In Vivo Pharmacokinetic Study
2.12. Biodistribution Study
2.13. HPLC Analysis
2.13.1. Assay Conditions
2.13.2. Preparation of Biological Samples
2.14. Statistical Analysis
3. Results
3.1. Evaluation of the Synergistic Effect of PTX, ETP, and RAPA
3.2. Physicochemical Characterization of PTX/ETP/RAPA-Loaded mPEG-pH-PCL Micelles
3.3. In Vitro pH-Sensitivity Assessment of PTX/ETP/RAPA-Loaded mPEG-pH-PCL Micelles
3.4. In Vitro Cytotoxicity Assay
3.5. In Vitro Clonogenic Assay
3.6. In Vitro Drug Release Assay
3.7. In Vivo Pharmacokinetic Study
3.8. Biodistribution Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padmanabhan, N.; Ushijima, T.; Tan, P. How to stomach an epigenetic insult: The gastric cancer epigenome. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; El-Serag, H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020, 18, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. Gastric cancer: Overview. Gastroenterol. Clin. N. Am. 2013, 42, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, C.; Wang, D.; Liang, J.; Guo, Y.; Zhu, Y.; Xia, J.; Qin, J.; Zhan, H.; Wang, J. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics 2019, 9, 4437–4449. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, J.; Ding, N.; Chen, W.; Chen, H.; Xue, M.; Chen, F.; Ni, J.; Wang, Z.; Lin, Z.; et al. Prodrug polymeric micelles integrating cancer-associated fibroblasts deactivation and synergistic chemotherapy for gastric cancer. J. Nanobiotechnol. 2021, 19, 381. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhu, L.; Zheng, K.; Liu, J.; Tian, P.; Hu, D.; Wang, Q.; Zuo, Q.; Ouyang, X.; Dai, Y.; et al. The design and synthesis of redox-responsive oridonin polymeric prodrug micelle formulation for effective gastric cancer therapy. J. Mater. Chem. B 2021, 9, 3068–3078. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Wang, J.H.; Gu, L.Y.; Yao, X.M.; Cai, F.Y.; Jing, M.; Li, X.T.; Ju, R.J. Dual variable of drug loaded micelles in both particle and electrical charge on gastric cancer treatment. J. Drug Target. 2020, 28, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Lee, K.-Y.; Hsu, N.-Y.; Chiang, Y.-T.; Yu, L.-Y.; Shen, Y.-A.; Lo, C.-L. A pH sensitive polymeric micelle for co-delivery of doxorubicin and α-TOS for colon cancer therapy. J. Mater. Chem. B 2017, 5, 5870–5880. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, Y.; Zhang, C.Y.; Fang, T. Co-delivery of paclitaxel and doxorubicin by pH-responsive prodrug micelles for cancer therapy. Int. J. Nanomed. 2020, 15, 3319. [Google Scholar] [CrossRef]
- Cho, H.; Lai, T.C.; Tomoda, K.; Kwon, G.S. Polymeric micelles for multi-drug delivery in cancer. AAPS Pharmscitech 2015, 16, 10–20. [Google Scholar] [CrossRef]
- Shin, H.J.; Jo, M.J.; Jin, I.S.; Park, C.-W.; Kim, J.-S.; Shin, D.H. Optimization and Pharmacokinetic Evaluation of Synergistic Fenbendazole and Rapamycin Co-Encapsulated in Methoxy Poly (Ethylene Glycol)-b-Poly (Caprolactone) Polymeric Micelles. Int. J. Nanomed. 2021, 16, 4873. [Google Scholar] [CrossRef]
- Kato, K.; Chin, K.; Yoshikawa, T.; Yamaguchi, K.; Tsuji, Y.; Esaki, T.; Sakai, K.; Kimura, M.; Hamaguchi, T.; Shimada, Y. Phase II study of NK105, a paclitaxel-incorporating micellar nanoparticle, for previously treated advanced or recurrent gastric cancer. Investig. New Drugs 2012, 30, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S. Taxol (paclitaxel): Mechanisms of action. Ann. Oncol. 1994, 5, S3–S6. [Google Scholar] [PubMed]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, E.; Osheroff, N. Etoposide, topoisomerase II and cancer. Curr. Med. Chem. Anticancer Agents 2005, 5, 363–372. [Google Scholar] [CrossRef]
- Dumont, F.J.; Su, Q. Mechanism of action of the immunosuppressant rapamycin. Life Sci. 1996, 58, 373–395. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, S.N. Rapamune (RAPA, rapamycin, sirolimus): Mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin. Biochem. 1998, 31, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, X.-C.; Xu, S.-F.; Xu, M. Paclitaxel and etoposide co-loaded polymeric nanoparticles for the effective combination therapy against human osteosarcoma. J. Nanobiotechnol. 2015, 13, 22. [Google Scholar] [CrossRef] [Green Version]
- Shafer, A.; Zhou, C.; Gehrig, P.A.; Boggess, J.F.; Bae-Jump, V.L. Rapamycin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and induction of apoptosis. Int. J. Cancer 2010, 126, 1144–1154. [Google Scholar] [CrossRef]
- Itamochi, H.; Oishi, T.; Shimada, M.; Sato, S.; Uegaki, K.; Naniwa, J.; Sato, S.; Nonaka, M.; Terakawa, N.; Kigawa, J. Inhibiting the mTOR pathway synergistically enhances cytotoxicity in ovarian cancer cells induced by etoposide through upregulation of c-Jun. Clin. Cancer Res. 2011, 17, 4742–4750. [Google Scholar] [CrossRef]
- Yildiz, R.; Kalender, M.E.; Dane, F.; Sevinc, A.; Gumus, M.; Camci, C.; Alici, S.; Kaya, A.O.; Yaman, E.; Ozturk, B.; et al. Docetaxel combined with oral etoposide as second-line treatment for advanced gastric carcinoma after failure of platinum- and fluoropyrimidine-based regimens. J. Oncol. Pharm. Pract. 2009, 16, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Fukamachi, H.; Kim, S.K.; Koh, J.; Lee, H.S.; Sasaki, Y.; Yamashita, K.; Nishikawaji, T.; Shimada, S.; Akiyama, Y.; Byeon, S.J.; et al. A subset of diffuse-type gastric cancer is susceptible to mTOR inhibitors and checkpoint inhibitors. J. Exp. Clin. Cancer Res. 2019, 38, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Awasthi, N.; Schwarz, M.A.; Schwarz, R.E. The dual PI3K/mTOR inhibitor NVP-BEZ235 enhances nab-paclitaxel antitumor response in experimental gastric cancer. Int. J. Oncol. 2013, 43, 1627–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.H.; Tam, Y.T.; Kwon, G.S. Polymeric micelle nanocarriers in cancer research. Front. Chem. Sci. Eng. 2016, 10, 348–359. [Google Scholar] [CrossRef]
- Jo, M.J.; Jo, Y.H.; Lee, Y.J.; Park, C.-W.; Kim, J.-S.; Hong, J.T.; Chung, Y.B.; Lee, M.K.; Shin, D.H. Physicochemical, pharmacokinetic, and toxicity evaluation of methoxy poly(ethylene glycol)-b-poly(d, l-Lactide) polymeric micelles encapsulating alpinumisoflavone extracted from unripe Cudrania tricuspidata fruit. Pharmaceutics 2019, 11, 366. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release Off. J. Control. Release Soc. 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Kwon, G.S.; Okano, T. Polymeric micelles as new drug carriers. Adv. Drug Deliv. Rev. 1996, 21, 107–116. [Google Scholar] [CrossRef]
- Yang, H.Y.; Jang, M.-S.; Gao, G.H.; Lee, J.H.; Lee, D.S. Construction of redox/pH dual stimuli-responsive PEGylated polymeric micelles for intracellular doxorubicin delivery in liver cancer. Polym. Chem. 2016, 7, 1813–1825. [Google Scholar] [CrossRef]
- Zhou, H.; Qi, Z.; Xue, X.; Wang, C. Novel pH-sensitive urushiol-loaded polymeric micelles for enhanced anticancer activity. Int. J. Nanomed. 2020, 15, 3851. [Google Scholar] [CrossRef]
- Bae, Y.; Fukushima, S.; Harada, A.; Kataoka, K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angew. Chem. (Int. Ed. Engl.) 2003, 42, 4640–4643. [Google Scholar] [CrossRef]
- Bui, Q.N.; Li, Y.; Jang, M.-S.; Huynh, D.P.; Lee, J.H.; Lee, D.S. Redox-and pH-sensitive polymeric micelles based on poly(β-amino ester)-grafted disulfide methylene oxide poly(ethylene glycol) for anticancer drug delivery. Macromolecules 2015, 48, 4046–4054. [Google Scholar] [CrossRef]
- Huang, X.; Liao, W.; Zhang, G.; Kang, S.; Zhang, C.Y. pH-sensitive micelles self-assembled from polymer brush (PAE-g-cholesterol)-b-PEG-b-(PAE-g-cholesterol) for anticancer drug delivery and controlled release. Int J. Nanomed. 2017, 12, 2215–2226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felber, A.E.; Dufresne, M.-H.; Leroux, J.-C. pH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates. Adv. Drug Deliv. Rev. 2012, 64, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Li, Z.Y.; Zhu, J.Y.; Han, K.; Zeng, Z.Y.; Hong, W.; Li, W.X.; Jia, H.Z.; Liu, Y.; Zhuo, R.X. Dual-pH sensitive charge-reversal polypeptide micelles for tumor-triggered targeting uptake and nuclear drug delivery. Small 2015, 11, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, Q.; Wang, J.; Chen, M.; Li, S.; Lin, Z.; Li, J. Tumor-targeted aggregation of pH-sensitive nanocarriers for enhanced retention and rapid intracellular drug release. Polym. Chem. 2014, 5, 5668–5679. [Google Scholar] [CrossRef]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Schmaljohann, D. Thermo-and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Wike-Hooley, J.; Haveman, J.; Reinhold, H. The relevance of tumour pH to the treatment of malignant disease. Radiother. Oncol. 1984, 2, 343–366. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef]
- Gao, G.H.; Li, Y.; Lee, D.S. Environmental pH-sensitive polymeric micelles for cancer diagnosis and targeted therapy. J. Control. Release Off. J. Control. Release Soc. 2013, 169, 180–184. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Yang, J.; Zhou, C.; Sun, J. pH-sensitive polymeric micelles triggered drug release for extracellular and intracellular drug targeting delivery. Asian J. Pharm. Sci. 2013, 8, 159–167. [Google Scholar] [CrossRef]
- Shao, J.; Zheng, D.; Jiang, Z.; Xu, H.; Hu, Y.; Li, X.; Lu, X. Curcumin delivery by methoxy polyethylene glycol–poly(caprolactone) nanoparticles inhibits the growth of C6 glioma cells. Acta Biochim. Biophys. Sin. 2011, 43, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Su, T.; Zhang, L.; Liu, R.; Wang, G.; He, B.; Gu, Z. Polymeric micelles with citraconic amide as pH-sensitive bond in backbone for anticancer drug delivery. Int. J. Pharm. 2014, 471, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation. Methods Mol. Biol. (Clifton N.J.) 2017, 1522, 17–22. [Google Scholar] [CrossRef]
- Xu, H.; Hou, Z.; Zhang, H.; Kong, H.; Li, X.; Wang, H.; Xie, W. An efficient Trojan delivery of tetrandrine by poly(N-vinylpyrrolidone)-block-poly(ε-caprolactone) (PVP-b-PCL) nanoparticles shows enhanced apoptotic induction of lung cancer cells and inhibition of its migration and invasion. Int J. Nanomed. 2014, 9, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Liang, Y.; Hao, N.; Xu, L.; Cheng, F.; Su, T.; Cao, J.; Gao, W.; Pu, Y.; He, B. A ROS-responsive polymeric micelle with a π-conjugated thioketal moiety for enhanced drug loading and efficient drug delivery. Org. Biomol. Chem. 2017, 15, 9176–9185. [Google Scholar] [CrossRef]
- Twentyman, P.R.; Luscombe, M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer 1987, 56, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Mikhail, A.S.; Eetezadi, S.; Allen, C. Multicellular tumor spheroids for evaluation of cytotoxicity and tumor growth inhibitory effects of nanomedicines in vitro: A comparison of docetaxel-loaded block copolymer micelles and Taxotere®. PLoS ONE 2013, 8, e62630. [Google Scholar] [CrossRef]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Berger, D.P.; Henss, H.; Winterhalter, B.R.; Fiebig, H.H. The clonogenic assay with human tumor xenografts: Evaluation, predictive value and application for drug screening. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1990, 1, 333–341. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S. A review of in vitro drug release test methods for nano-sized dosage forms. Adv. Pharm. 2014, 2014, 304757. [Google Scholar] [CrossRef] [Green Version]
- Modi, S.; Anderson, B.D. Determination of drug release kinetics from nanoparticles: Overcoming pitfalls of the dynamic dialysis method. Mol. Pharm. 2013, 10, 3076–3089. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Park, S.H.; Jeong, S.W.; Park, C.-W.; Han, K.; Chung, Y.B. Hepatic uptake of epirubicin by isolated rat hepatocytes and its biliary excretion after intravenous infusion in rats. Arch. Pharm. Res. 2014, 37, 1599–1606. [Google Scholar] [CrossRef]
- Aw, M.S.; Kurian, M.; Losic, D. Polymeric micelles for multidrug delivery and combination therapy. Chem. Eur. J. 2013, 19, 12586–12601. [Google Scholar] [CrossRef]
- Jo, M.J.; Jin, I.S.; Park, C.-W.; Hwang, B.Y.; Chung, Y.B.; Kim, J.-S.; Shin, D.H. Revolutionizing technologies of nanomicelles for combinatorial anticancer drug delivery. Arch. Pharm. Res. 2020, 43, 100–109. [Google Scholar] [CrossRef]
- Huh, K.M.; Lee, S.C.; Cho, Y.W.; Lee, J.; Jeong, J.H.; Park, K. Hydrotropic polymer micelle system for delivery of paclitaxel. J. Control. Release Off. J. Control. Release Soc. 2005, 101, 59–68. [Google Scholar] [CrossRef]
- Jiang, H.; Pei, L.; Liu, N.; Li, J.; Li, Z.; Zhang, S. Etoposide-loaded nanostructured lipid carriers for gastric cancer therapy. Drug Deliv. 2016, 23, 1379–1382. [Google Scholar] [CrossRef] [Green Version]
- Yáñez, J.A.; Forrest, M.L.; Ohgami, Y.; Kwon, G.S.; Davies, N.M. Pharmacometrics and delivery of novel nanoformulated PEG-b-poly (ε-caprolactone) micelles of rapamycin. Cancer Chemother. Pharmacol. 2008, 61, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Liu, G.; Kang, J.; Niu, M.; Wang, Z.; Wang, H.; Ma, J.; Wang, X. Paclitaxel nanosuspensions coated with P-gp inhibitory surfactants: I. Acute toxicity and pharmacokinetics studies. Colloids Surf. B Biointerfaces 2013, 111, 277–281. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, D.W.; Shim, Y.H.; Bang, J.S.; Oh, H.S.; Kim, S.W.; Seo, M.H. In vivo evaluation of polymeric micellar paclitaxel formulation: Toxicity and efficacy. J. Control. Release Off. J. Control. Release Soc. 2001, 72, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, A.A.A.; Quach, J.R.C.; Bundgaard, C.; Steffansen, B.; Holm, R.; Nielsen, C.U. Polysorbate 20 alters the oral bioavailability of etoposide in wild type and mdr1a deficient Sprague-Dawley rats. Int. J. Pharm. 2018, 543, 352–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesh, P.; Harisudhan, T.; Choudhury, H.; Mullangi, R.; Srinivas, N.R. Pharmacokinetics of etoposide in rats with uranyl nitrate (UN)-induced acute renal failure (ARF): Optimization of the duration of UN dosing. Eur. J. Drug Metab. Pharm. 2007, 32, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhang, L.; Niu, Y.; Fan, D.; Wu, X.; Tang, X.; Cai, C. A stable and practical etoposide-containing intravenous long-/medium-chain triglycerides-based lipid emulsion formulation: Pharmacokinetics, biodistribution, toxicity, and antitumor efficacy. Expert Opin. Drug Deliv. 2013, 10, 559–571. [Google Scholar] [CrossRef]
- Wang, F.; Yang, K.; Wang, Z.; Ma, Y.; Gutkind, J.S.; Hida, N.; Niu, G.; Tian, J. Combined image guided monitoring the pharmacokinetics of rapamycin loaded human serum albumin nanoparticles with a split luciferase reporter. Nanoscale 2016, 8, 3991–4000. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Zhu, M.; Zhou, S.; Feng, W.; Chen, H. Rapamycin-loaded mPEG-PLGA nanoparticles ameliorate hepatic steatosis and liver injury in non-alcoholic fatty liver disease. Front. Chem. 2020, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Q.; Zhu, W.-T.; Lin, C.-Y.; Yuan, Z.-W.; Li, Z.-H.; Yan, P.-K. Delivery of rapamycin by liposomes synergistically enhances the chemotherapy effect of 5-fluorouracil on colorectal cancer. Int. J. Nanomed. 2021, 16, 269. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Liao, J.; Song, Y.; Liu, C.; Li, D.; Zheng, H.; Lu, B. Dual-drug delivery based charge-conversional polymeric micelles for enhanced cellular uptake and combination therapy. Polym. Chem. 2019, 10, 5879–5893. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Nyström, A.M. Endocytic uptake and intracellular trafficking of bis-MPA-based hyperbranched copolymer micelles in breast cancer cells. Biomacromolecules 2012, 13, 3814–3822. [Google Scholar] [CrossRef]
- Manjili, H.K.; Malvandi, H.; Mousavi, M.S.; Attari, E.; Danafar, H. In vitro and in vivo delivery of artemisinin loaded PCL–PEG–PCL micelles and its pharmacokinetic study. Artif. Cells Nanomed. Biotechnol. 2018, 46, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qu, G.; Sun, Y.; Wu, X.; Yao, Z.; Guo, Q.; Ding, Q.; Yuan, S.; Shen, Z.; Ping, Q. Pharmacokinetics, biodistribution, efficacy and safety of N-octyl-O-sulfate chitosan micelles loaded with paclitaxel. Biomaterials 2008, 29, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Li, X.; Sang, Z.; Mei, L.; Yang, T.; Li, Z.; Zhou, L.; Zheng, Y.; He, G.; Guo, G. Improving the pharmacokinetics and tissue distribution of pyrinezolid by self-assembled polymeric micelles. Colloids Surf. B 2017, 156, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Chen, Y.C.; Lin, C.C.; Chen, C.F.; Chen, J.W. Characterization of pegylated copolymeric micelles and in vivo pharmacokinetics and biodistribution studies. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77, 188–194. [Google Scholar] [CrossRef]







| PTX:ETP:RAPA (Weight Ratio) | IC50 (nM) | CI Value | ||
|---|---|---|---|---|
| PTX | ETP | RAPA | ||
| 2:2:1 | 1.32 ± 0.44 | 1.92 ± 0.63 | 0.62 ± 0.20 | 0.06 ± 0.02 |
| 1:1:1 | 1.20 ± 0.09 | 1.73 ± 0.13 | 1.12 ± 0.09 | 0.06 ± 0.00 |
| Formulation | Amount of Polymer Used (mg) | Encapsulation Efficiency (EE %) | Drug Loading (DL %) | Particle Size (nm) | Poly-Dispersity Index (PDI) | Zeta Potential (mV) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| PTX | ETP | RAPA | PTX | ETP | RAPA | |||||
| PTX:ETP:RAPA (2:2:1) | 150 | 64.8 ± 1.85 | 67.3 ± 1.41 | 70.3 ± 2.71 | 2.49 ± 0.07 | 2.59 ± 0.05 | 1.38 ± 0.05 | 35.0 ± 0.24 | 0.03 ± 0.70 | −0.22 ± 0.03 |
| (A) | |||||
| Time (h) | 0 | 2 | 4 | 6 | 8 |
| Particle size (nm) | 35.0 ± 0.24 | 41.0 ± 0.71 | 48.7 ± 3.04 | 66.9 ± 23.4 | 433,501 ± 612,891 |
| PDI | 0.03 ± 0.70 | 0.22 ± 0.46 | 0.28 ± 1.15 | 0.26 ± 4.58 | 1.92 ± 247 |
| Zeta potential (mV) | −0.22 ± 0.03 | −2.90 ± 0.70 | −0.33 ± 0.20 | −0.13 ± 0.03 | −5.22 ± 8.26 |
| (B) | |||||
| Time (h) | 0 | 2 | 4 | 6 | 8 |
| Particle size (nm) | 35.1 ± 0.31 | 41.4 ± 2.30 | 50.4 ± 3.36 | 38,5002 ± 168,486 | 118,618 ± 166,924 |
| PDI | 0.04 ± 1.11 | 0.23 ± 1.48 | 0.26 ± 2.16 | 1.07 ± 111 | 0.75 ± 69.6 |
| Zeta potential (mV) | −0.08 ± 0.18 | −1.30 ± 0.58 | 0.55 ± 0.85 | −0.17 ± 0.15 | −0.03 ± 0.08 |
| Parameters | PTX in Combination Solution | PTX in Combination Micelle | ETP in Combination Solution | ETP in Combination Micelle | RAPA in Combination Solution | RAPA in Combination Micelle |
|---|---|---|---|---|---|---|
| Dose (µg∙kg−1) | 10,000 | 10,000 | 10,000 | 10,000 | 5000 | 5000 |
| AUC (min∙µg∙mL−1) | 821 ± 127 | 1300 ± 314 | 445 ± 231 | 1331 ± 221 | 87.4 ± 15.4 | 206 ± 40.5 |
| CLt (mL∙kg−1∙min) | 12.4 ± 1.98 | 8.01 ± 1.98 | 29.1 ± 19.8 | 7.66 ± 1.34 | 58.3 ± 9.37 | 24.9 ± 5.31 |
| Vd (mL∙kg−1) | 340 ± 52.1 | 264 ± 17.3 | 579 ± 147 | 403 ± 56.3 | 1245 ± 145 | 783 ± 327 |
| t1/2 (min) | 19.1 ± 1.70 | 23.9 ± 7.05 | 16.3 ± 5.62 | 37.1 ± 7.00 | 15.1 ± 3.39 | 21.4 ± 6.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, M.J.; Shin, H.J.; Yoon, M.S.; Kim, S.Y.; Jin, C.E.; Park, C.-W.; Kim, J.-S.; Shin, D.H. Evaluation of pH-Sensitive Polymeric Micelles Using Citraconic Amide Bonds for the Co-Delivery of Paclitaxel, Etoposide, and Rapamycin. Pharmaceutics 2023, 15, 154. https://doi.org/10.3390/pharmaceutics15010154
Jo MJ, Shin HJ, Yoon MS, Kim SY, Jin CE, Park C-W, Kim J-S, Shin DH. Evaluation of pH-Sensitive Polymeric Micelles Using Citraconic Amide Bonds for the Co-Delivery of Paclitaxel, Etoposide, and Rapamycin. Pharmaceutics. 2023; 15(1):154. https://doi.org/10.3390/pharmaceutics15010154
Chicago/Turabian StyleJo, Min Jeong, Hee Ji Shin, Moon Sup Yoon, Seo Yeon Kim, Chae Eun Jin, Chun-Woong Park, Jin-Seok Kim, and Dae Hwan Shin. 2023. "Evaluation of pH-Sensitive Polymeric Micelles Using Citraconic Amide Bonds for the Co-Delivery of Paclitaxel, Etoposide, and Rapamycin" Pharmaceutics 15, no. 1: 154. https://doi.org/10.3390/pharmaceutics15010154