Pharmacokinetic Study of Intranasal Dexamethasone and Methylprednisolone Compared with Intravenous Administration: Two Open-Label, Single-Dose, Two-Period, Two-Sequence, Cross-Over Study in Healthy Volunteers
Abstract
:1. Introduction
2. Materials and Methods
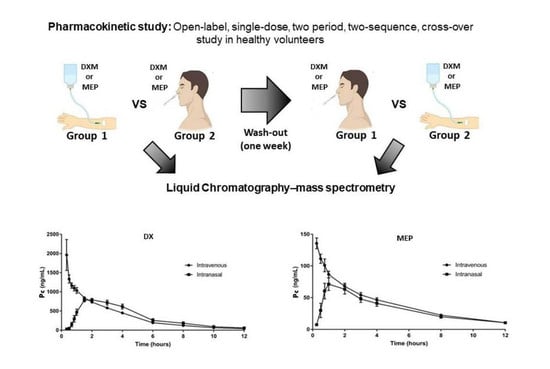
2.1. Study Design
2.2. Volunteers
2.3. DXM and MEP Dosing
2.4. Bioanalytic Method: DXM
2.5. Bioanalytic Method: MEP
2.6. Pharmacokinetic and Statistical Analysis
2.7. Tolerability Assessment of IN Drug Administration
3. Results
3.1. Demographic Data of Volunteers
3.2. Pharmacokinetics Assessment
3.3. Tolerability Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Departamento de Neurología, Instituto Nacional de Neurología y Neurocirugía (INNN), Av. Insurgentes Sur 3877, La Fama, Tlalpan, Ciudad de Mexico 14269, Mexico; gracielacardenas@yahoo.com.mx (G.C.); lau.torresaraujo@gmail.com (L.V.T.A.); drbarajas@hotmail.es (R.L.B.C.); alder_30@hotmail.com (A.C.H.)
- Instituto Nacional de Cardiología Ignacio Chávez and Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de Mexico, Juan Badiano No. 1, Col. Sección XVI, Tlalpan, Ciudad de Mexico 14080, Mexico; maria@iibiomedicas.unam.mx (M.C.-C); ajordanrios@gmail.com; (A.J.-R.); manlio.marquez@gmail.com (M.F.M.M.); qfbyoana@gmail.com (Y.L.)
- Hospital General de Mexico Dr. Eduardo Liceaga, Dr. Balmis 148, Doctores, Cuauhtémoc, Ciudad de Mexico 06720, Mexico; Unidad de Investigación UNAM-INC; anaesga@hotmail.com (A.M.E.); anicamalagon@yahoo.com.mx (D.A.M.); hernandezjoselin@hotmail.com (J.H.R.); leonhmireya@gmail.com (M.L.H.M.)
- Facultad de Medicina, Universidad Nacional Autónoma de Mexico, Ciudad de Mexico 04510, Mexico; rmwong@unam.mx
- Unidad Temporal COVID-19, Centro Citibanamex. Avenida del Conscripto 311, Lomas de Sotelo, Miguel Hidalgo 11200, CDMX, Mexico; luisest01@hotmail.com (L.E.G.R.); karentibet.v@gmail.com (K.I.C.); quiqui7@hotmail.es (E.G.V.); mariana.rdlc@gmail.com (M.R.C.); a.chinney.h@gmail.com (A.C.H.)
- Hospital Militar, Secretaría de la Defensa Nacional Periférico Blvrd Manuel Ávila Camacho s/n, Militar, Miguel Hidalgo, Ciudad de Mexico 11200, CDMX, Mexico; kmq.kmq5@gmail.com (Q.M.A.); amoncivais11@gmail.com; sehedi@yahoo.com.mx (S.H.D.); rosaliazeron_1981@hotmail.com (A.R.Z.M.); adyta0@hotmail.com (AM-C.); drivanmartinez@icloud.com (I.N.M.S.); edherzliche@hotmail.com (E.B.S.); afp_red01@hotmail.com (A.F.P.); sara.delarosa@gmail.com(P.S.H.H.); rafael.aguilar@gmail.com (R.I.A.R.); danimure95@gmail.com (D.M.R.); rodrigodlrio@gmail.com (L.R.R.A.); alfarobonillarogelioantonio@gmail.com (R.A.A.B.)
- Departamento de Inmunología, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de Mexico, Ciudad de Mexico 04510, Mexico; cruz.joss0308@gmail.com (J.C.); leonorhh@iibiomedicas.unam.mx (L.H.); noraalma@iibiomedicas.unam.mx (N.A.F.); marysel_01@yahoo.com.mx (M.H.); eespindola@iibiomedicas.unam.mx (E.E.-A.); dragg412@gmail.com (J.A.H.A.); rolguinalor@iibiomedicas.unam.mx (R.O.A.); andraamyris@iibiomedicas.unam.mx (S.O.F.); afuentes@iibiomedicas.unam.mx (A.F.R.); rbobes@iibiomedicas.unam.mx (R.J.B.); soldevi@unam.mx (G.S.); gladis@unam.mx (G.F.); laclettejp@gmail.com (J.P.L.); edda@unam.mx (E.S.)
- Unidad de Desarrollo e Investigación en Bioprocesos, Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional, Prolongación de Carpio y Plan de Ayala S/N, Col. Casco de Santo Tomas, Del. Miguel Hidalgo, Ciudad de Mexico 11340, Mexico; smpt.2011@hotmail.com (M.T.-P.)
- Instituto de Diagnóstico y Referencia Epidemiológicos Dr. Manuel Martínez Báez, Francisco de Miranda 177, Lomas de Plateros, Álvaro Obregón, Ciudad de Mexico 01480, Mexico; quilaz@yahoo.com (G.M.)
- Facultad de Química, Universidad Nacional Autónoma de Mexico, Ciudad de Mexico 04510, Mexico; helgi@unam.mx (H.J.)
- Universidad Autónoma de San Luis Potosí, Álvaro Obregón 64, Col. Centro, C.P. 78000, San Luis Potosí, San Luis Potosí, Mexico; rosales.s@uaslp.mx (S.R.M.)
- Facultad de Medicina, Universidad Autónoma del Estado de Morelos, Avenida Universidad No. 1001, Chamilpa, Cuernavaca 62209, Morelos, Mexico; gabyrosas62@hotmail.com (G.R.)
- Unidad de Investigación, Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Autónoma de Mexico, Ciudad de Mexico 04520, Mexico; paque81@hotmail.com (J.T.-C.)
- Institute of Physiology and Pathophysiology, Emil-Mannkopff-Straße 2 u, 35037 Marburg, Germany; besedovs@staff.uni-marburg.de (H.B.)
- Departamento de Fisiología, Biofísica y Neurociencias, Centro de Investigación y Estudios Avanzados del Instituto Politécnico Nacional, Av. Instituto Politécnico Nacional 2508, San Pedro Zacatenco, Gustavo A. Madero, Ciudad de Mexico 07360, Mexico; mromano@fisio.cinvestav.mx (M.C.R.)
- University of Texas Rio Grande Valley—UTRGV, 1201 W University Dr, Edinburg, TX 78539, USA; juan.lopezalvarenga@utrgv.edu (J.A.L.)
References
- Barnes, P.J. Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. J. Clin. Sci. 1998, 94, 557–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobile-Orazio, E.; Cocito, D.; Jann, S.; Uncini, A.; Beghi, E.; Messina, P.; Antonini, G.; Fazio, R.; Gallia, F.; Schenone, A.; et al. IMC Trial Group. Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: A randomized controlled trial. Lancet Neurol. 2012, 11, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid regulation of inflammation and its functional correlates: From HPA axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci. 2012, 126, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, X.; Chen, S.; Xiao, Y.; Zhuang, W. Oral versus intravenous methylprednisolone for the treatment of multiple treatment of multiple sclerosis relapses: A meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0188644. [Google Scholar] [CrossRef] [Green Version]
- Shimba, A.; Ikuta, K. Control of immunity by glucocorticoids in health and disease. Semin. Immunopathol. 2020, 42, 669–680. [Google Scholar] [CrossRef]
- Bleier, B.S.; Paulson, D.P.; O’Malley, B.W.; Li, D.; Palmer, J.N.; Chiu, A.G.; Cohen, N.A. Chitosan glycerophosphate-based semirigid dexamethasone eluting biodegradable stent. Am. J. Rhinol. Allergy 2009, 23, 76–79. [Google Scholar] [CrossRef]
- Bassett, D.; Hirata, F.; Gao, X.; Kannan, R.; Kerr, J.; Doyon-Reale, N.; Wilson, S.; Lieh-Lai, M. Reversal of methylprednisolone effects in allergen-exposed female BALB/c mice. J. Toxicol. Environ. Health A 2010, 73, 711–724. [Google Scholar] [CrossRef]
- Hommes, O.R.; Barkhof, F.; Jongen, P.J.; Frequin, S.T. Methylprenisolone treatment in multiple sclerosis: Effect of treatment pharmacokinetics, future. Mult. Scler. 1996, 1, 327–328. [Google Scholar] [CrossRef]
- Börü, Ü.T.; Erdogän, H.; Alp, R.; Taşdemir, M.; Yildirim, S.; Bilgiç, A.; Duman, A.; Arslan, A. Treatment of chronic inflammatory demyelinating polyneuropathy with high dose intravenous methylprednisolone monthly for five years: 10-year follow up. Clin. Neurol. Neurosurg. 2014, 118, 89–93. [Google Scholar] [CrossRef]
- Ratzer, R.; Iversen, P.; Börnsen, L.; Dyrby, T.B.; Romme Christensen, J.; Ammitzboll, C.; Madsen, C.G.; Garde, E.; Lyksborg, M.; Andersen, B.; et al. Monthly oral methylprednisolone pulse treatment in progressive multiple sclerosis. Mult. Scler. 2016, 22, 926–934. [Google Scholar] [CrossRef]
- Toledo, A.; Osorio, R.; Matus, C.; Martinez Lopez, Y.; Ramirez Cruz, N.; Sciutto, E.; Fragoso, G.; Arauz, A.; Carrillo-Mezo, R.; Fleury, A. Human extraparenchymal neurocysticercosis: The control of inflammation favors the host…but also the parasite. Front. Immunol. 2018, 16, 2652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iorgulescu, J.B.; Gokhale, P.C.; Speranza, M.C.; Eschle, B.K.; Poitras, M.J.; Wilkens, M.K.; Soroko, K.M.; Chhoeu, C.; Knott, A.; Gao, Y.; et al. Concurrent dexamethasone limits the clinical benefit of immune checkpoint blockade in glioblastoma. Clin. Cancer Res. 2021, 27, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.L.; Pariante, C.M.; Jamel, S.; Thomas, S.A. Central nervous system (CNS) delivery of glucocorticoids is fine-tuned by saturable transporters at the blood-CNS barriers and nonbarrier regions. Endocrinology 2010, 151, 5294–5305. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.H. P-glycoprotein, a gatekeeper in the blood-brain barrier. Adv. Drug Deliv. Rev. 1999, 36, 179–194. [Google Scholar] [CrossRef]
- Pariante, C.M. Glucocorticoid receptor function in vitro in patients with major depression. Stress 2004, 7, 209–219. [Google Scholar] [CrossRef]
- Schinkel, A.H.; Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv. Drug Deliv. Rev. 2003, 55, 3–29. [Google Scholar] [CrossRef]
- Thiebaut, F.; Tsuruo, T.; Hamada, H.; Gottesman, M.M.; Pastan, I.; Willingham, M.C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 7735–7738. [Google Scholar] [CrossRef] [Green Version]
- Graff, C.L.; Pollack, G.M. P-Glycoprotein attenuates brain uptake of substrates after nasal instillation. Pharm. Res. 2003, 20, 1225–1230. [Google Scholar] [CrossRef]
- Crowe, A.; Tan, A.M. Oral and inhaled corticosteroids: Differences in P-glycoprotein (ABCB1) mediated efflux. Toxicol. Appl. Pharmacol. 2012, 260, 294–302. [Google Scholar] [CrossRef]
- Defer, G.L.; Barré, J.; Ledudal, P.; Tillement, J.P.; Degos, J.D. Methylprednisolone infusion during acute exacerbation of MS: Plasma and CSF concentrations. Eur. Neurol. 1995, 35, 143–148. [Google Scholar] [CrossRef]
- Barth, J.; Wnkler, J.; Schummann, R.; Nagaraja, N.V.; Madabushi, R.; Balbach, S.; Derendorf, H.; Möllmann, H.; Möllenhoff, G. Population pharmacokinetics of methylprednisolone in accident victims with spinal cord injury. Int. J. Clin. Pharmacol. Ther. 2004, 42, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Osorio, I.N.; Espinosa, A.; Giraldo Velázquez, M.; Padilla, P.; Bárcena, B.; Fragoso, G.; Jung-Cook, H.; Besedovsky, H.; Meneses, G.; Sciutto Conde, E.L. Nose-to-brain delivery of dexamethasone: Biodistribution studies in mice. J. Pharmacol. Exp. Ther. 2021, 378, 244–250. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Bokatyi, A.N.; Golovkin, A.S.; Kudryavtsev, I.V.; Serebryakova, M.K.; Trulioff, A.S.; Dubrovskii, Y.A.; Skorik, Y.A. Synthesis and characterization of novel succinyl chitosan-dexamethasone conjugates for potential intravitreal dexamethasone delivery. Int. J. Mol. Sci. 2021, 22, 10960. [Google Scholar] [CrossRef] [PubMed]
- Dubashynskaya, N.; Bokatyi, A.N.; Skorik, Y.A. Dexamethasone conjugates: Synthetic approaches and medical prospects. Biomedicines 2021, 9, 341. [Google Scholar] [CrossRef]
- Frey, W.H., II. (WO/1991/007947) Neurologic Agents for Nasal Administration to the Brain (Priority Date 51289); World Intellectual Property Organization: Geneva, Switzerland, 1991; Available online: http://www.wipo.int/pctdb/en/wo.jsp?wo=1991007947&IA=WO1991007947&DISPLAY=CLAIMS (accessed on 9 November 2022).
- Meneses, G.; Gevorkian, G.; Florentino, A.; Bautista, M.A.; Espinosa, A.; Acero, G.; Díaz, G.; Fleury, A.; Osorio, I.N.P.; del Rey, A.; et al. Intranasal delivery of dexamethasone efficiently controls LPS-induced murine neuroinflammation. Clin. Exp. Immunol. 2017, 190, 304–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rassy, D.; Bárcena, B.; Pérez-Osorio, I.N.; Espinosa, A.; Peón, A.N.; Terrazas, L.I.; Meneses, G.; Besedovsky, H.O.; Fragoso, G.; Sciutto, E. Intranasal methylprednisolone effectively reduces neuroinflammation in mice with experimental autoimmune encephalitis. J. Neuropathol. Exp. Neurol. 2020, 79, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Meneses, G.; Chavarría, A.; Mancilla, R.; Pedraza-Chaverri, J.; Fleury, A.; Bárcena, B.; Pérez-Osorio, I.N.; Besedovsky, H.; Arauz, A.; et al. Intranasal dexamethasone reduces mortality and brain damage in a mouse experimental ischemic stroke model. Neurotherapeutics 2020, 17, 1907–1918. [Google Scholar] [CrossRef]
- Giraldo-Velásquez, M.F.; Pérez-Osorio, N.I.; Espinosa-Cerón, A.; Barcena, B.M.; Calderón-Gallegos, A.; Fragoso, G.; Torres-Ramos, M.; Páez-Martínez, N.; Sciutto, E. Intranasal methylprednisolone ameliorates neuroinflammation induced by chronic toluene exposure. Pharmaceutics 2022, 14, 1195. [Google Scholar] [CrossRef]
- Pérez-Osorio, I.N. Distribución de Glucocorticoides en el Sistema Nervioso Central Administrados por vía Intranasal. Master’s Thesis, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2019. Available online: https://ru.dgb.unam.mx/handle/DGB_UNAM/TES01000794829 (accessed on 9 November 2022).
- Lara-Espinosa, J.V.; Arce-Aceves, M.F.; Mata-Espinosa, D.; Barrios-Payán, J.; Marquina-Castillo, B.; Hernandez-Pando, R. The therapeutic effect of intranasal administration of dexamethasone in neuroinflammation induced by experimental pulmonary tuberculosis. Int. J. Mol. Sci. 2021, 22, 5997. [Google Scholar] [CrossRef]
- Menter, T.; Haslbauer, J.D.; Niendhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. A Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef]
- Yamaoko-Tojo, M. Endothelial glycocalyx damage as a systemic inflammatory microvascular endotheliopathy in COVID-19. Biomed. J. 2020, 43, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Papamanoli, A.; Yoo, J.; Grewal, P.; Predun, W.; Hotelling, J.; Jacob, R.; Mojahedi, A.; Skopicki, H.A.; Mansour, M.; Marcos, L.A.; et al. High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. Eur. J. Clin. Investig. 2021, 51, e13458. [Google Scholar] [CrossRef]
- Hasan, S.S.; Know, C.S.; Mustafa, Z.E.; Merchant, H.A. Does Methylprednisolone reduce mortality risk in hospitalized COVID-19 patients? A meta-analysis of randomized control trials. Expert. Rev. Respir. Med. 2021, 15, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.J.; Wu, C.; Mehta, N.; Wald-Dickler, N.; Yang, W.; Qiao, R. A comparison of methyl prednisolone and dexamethasone in intensive care patients with COVID-19. J. Intensive Care Med. 2021, 36, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Pinzon, M.; Ortiz, S.; Holguin, H.; Betancur, J.F.; Cardona Arango, D.; Laniado, H.; Arias Arias, C.; Muñoz, B.; Quiceno, J.; Jaramillo, D. Dexamethasone vs methylprednisolone high dose for COVID-19 pneumonia. PLoS ONE 2021, 16, e0252057. [Google Scholar] [CrossRef]
- Delliere, S.; Dudoignon, E.; Fondil, S.; Voicu, S.; Collet, M.; Oillic, P.A.; Salmona, M.; Dépret, F.; Ghelfenstein-Ferreira, T.; Plaud, B. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: A French multicentric retrospective cohort. Clin. Microbiol. Infect. 2020, 27, e1–e790. [Google Scholar] [CrossRef]
- Mehta, S.; Pandey, A. Rhino-Orbital mucormycosis associated with COVID-19. Cureus 2020, 12, e10726. [Google Scholar] [CrossRef]
- Gandra, S.; Ram, S.; Levitz, S.M. The “Black fungus” in india: The emerging syndemic of COVID-19 -associated mucormycosis. Ann. Intern. Med. 2021, 174, 1301–1302. [Google Scholar] [CrossRef]
- Meijer, E.F.J.; Doffenhoff, A.S.M.; Hoiting, O.; Meis, J.F. COVID-19-associated pulmonary aspergillosis: A prospective single-center dual case series. Mycoses 2021, 64, 457–464. [Google Scholar] [CrossRef]
- Said Ahmed, W.M.; Elsherbini, A.M.; Elsherbiny, N.M.; El-Sherbiny, M.; Ramzy, N.I.; Arafa, A.F. Maxillary mucormycosis osteomyelitis in post COVID-19 patients: A series of fourteen cases. Diagnostics 2021, 11, 2050. [Google Scholar] [CrossRef] [PubMed]
- Boroujeni, M.E.; Simani, L.; Bluyssen, H.A.R.; Samadikhah, H.R.; Zamanlui Benisi, S.; Hassani, S.; Akbari Dilmaghani, N.; Fathi, M.; Vakili, K.; MahmoudiasI, G.R. Inflammatory response leads to neuronal death in human post-mortem cerebral cortex in patients with COVID-19. ACS Chem. Neurosci. 2021, 12, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Spehake, J.P.; Schröder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929. [Google Scholar] [CrossRef]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood-brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef]
- Hochhaus, G.; Barth, J.; al-Fayoumi, S.; Suarez, S.; Derendorf, H.; Hochhaus, R.; Möllmann, H. Pharmacokinetics and pharmacodynamics of dexamethasone sodium-m-sulfobenzoate (DS) after intravenous and intramuscular administration: A comparison with dexamethasone phosphate (DP). J. Clin. Pharmacol. 2001, 41, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Bashir, Q.; Acosta, M. Comparative safety, bioavailability, and pharmacokinetics of oral dexamethasone, 4-mg and 20-mg tablets, in healthy volunteers under fasting and fed conditions: A randomized open-label, 3-way crossover study. Clin. Lymphoma Myeloma Leuk. 2020, 20, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Jusko, W.J. Across-species meta-analysis of dexamethasone pharmacokinetics utilizing allometric and scaling modeling approaches. Biopharm. Drug Dispos. 2021, 42, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Queckenberg, C.; Wachall, B.; Erlinghagen, V.; Di Gion, P.; Tomalik-Scharte, D.; Tawab, M.; Gerbeth, K.; Fuhr, U. Pharmacokinetics, pharmacodynamics, and comparative bioavailability of single, oral 2-mg doses of dexamethasone liquid and tablet formulations: A randomized, controlled, crossover study in healthy adult volunteers. Clin. Ther. 2011, 33, 1831–1841. [Google Scholar] [CrossRef]
- Toledo, A.; Amato, C.; Clarke, N.; Teitz, R.; Salo, D. Injectable dexamethasone sodium phosphate administered orally? A pharmacokinetic analysis of a common emergency department practice. J. Pediatr. Pharmacol. Ther. 2015, 20, 105–111. [Google Scholar] [CrossRef]
- Antal, E.J.; Wright, C.E., III; Gillespie, W.R.; Albert, K.S. Influence of route of administration on the pharmacokinetics of methylprednisolone. J. Pharmacokinetic. Biopharm. 1983, 11, 561–576. [Google Scholar] [CrossRef]
- Spoorenberg, S.M.; Deneer, V.H.; Grutters, J.C.; Pulles, A.E.; Voorn, G.P.; Rijkers, G.T.; Bos, W.J.; Van de Garde, E.M. Pharmacokinetics of oral vs. intravenous dexamethasone in patients hospitalized with community-acquired pneumonia. Br. J. Clin. Pharmacol. 2014, 78, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Narang, P.K.; Wilder, R.; Chatterji, D.C.; Yeager, R.L.; Gallelli, J.F. Systemic bioavailability and pharmacokinetics of methylprednisolone in patients with rheumatoid arthritis following ‘high-dose’ pulse administration. Biopharm. Drug Dispos. 1983, 4, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Czock, D.; Keller, F.; Rasche, F.M.; Häussler, U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin. Pharmacokinet. 2005, 44, 61–98. [Google Scholar] [CrossRef]
- Geister, U.; Guserle, R.; Bungers, E.; Schaarschmidt, D.; Doser, K. Bioavailability investigation of two different oral formulations of methylprednisolone. Arzneimittelforschung 2000, 50, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Al-Habet, S.M.; Rogers, H.J. Methylprednisolone pharmacokinetics after intravenous and oral administration. Br. J. Clin. Pharmacol. 1989, 27, 285–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerni, L.; Biancotto, G.; Tondolo, A.; Bogoni, P. Dexamethasone and clenbuterol detection by enzyme immunoassay in bovine liver tissue: A new multiresidue extraction procedure. Food Agric. Immunol. 1998, 10, 307–315. [Google Scholar] [CrossRef]
- Yun-Kyoung, S.; Jeong-Sook, P.; Jin-Ki, K.; Chong-Kook, K. HPLC determination of dexamethasone in human plasma. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 2293–2306. [Google Scholar] [CrossRef]
- Régina, A.; Romero, A.; Greenwood, J.; Adamson, P.; Bourre, J.M.; Couraud, P.O.; Roux, F. Dexamethasone regulation of P-glycoprotein activity in an immortalized rat brain endothelia cell line, GPNT. J. Neurochem. 1999, 73, 1954–1963. [Google Scholar]
- Varrone, A.; Bundgaard, C.; Bang-Andersen, B. PET as a translational tool in drug development for neuroscience compounds. Clin. Pharmacol. Ther. 2022, 111, 774–785. [Google Scholar] [CrossRef]
- Guo, Y.; Bera, H.; Shi, C.; Zhang, L.; Cun, D.; Yang, M. Pharmaceutical strategies to extend pulmonary exposure of inhaled medicines. Acta Pharm. Sin. B 2021, 11, 2565–2584. [Google Scholar] [CrossRef]
- Cárdenas, G.; Chávez-Canales, M.; Espinosa, A.M.; Jordán-Rios, A.; Malagon, D.A.; Murillo, M.F.M.; Araujo, L.V.T.; Campos, R.L.B.; Wong-Chew, R.M.; González, L.E.R.; et al. Intranasal dexamethasone: A new clinical trial for the control of inflammation and neuroinflammation in COVID-19 patients. Trials 2022, 23, 148. [Google Scholar] [CrossRef] [PubMed]


| Subject | Gender | Age (Years) | Scholarship (Years) | Weight (kg) | Height (cm) | BMI (kg/m2) | Sequence |
|---|---|---|---|---|---|---|---|
| 1 | Female | 25 | 9 | 52 | 162 | 19.8 | B-A |
| 2 | Male | 30 | 17 | 69 | 170 | 23.9 | A-B |
| 3 | Female | 42 | 12 | 58 | 158 | 23.2 | B-A |
| 4 | Female | 28 | 17 | 67 | 172 | 22.6 | A-B |
| 5 | Male | 39 | 17 | 64 | 175 | 20.9 | A-B |
| 6 | Male | 23 | 16 | 72 | 172 | 24.3 | B-A |
| 7 | Female | 31 | 9 | 60 | 156 | 24.7 | A-B |
| 8 | Male | 25 | 14 | 71 | 172 | 24.0 | B-A |
| Average | 30.38 | 13.88 | 64.13 | 167 | 22.93 | ||
| Standard Deviation | 6.84 | 3.48 | 7.0 | 7.00 | 1.73 | ||
| % CV | 22.53 | 25.10 | 10.91 | 4.38 | 7.55 | ||
| Subject | Gender | Age (Years) | Scholarship (Years) | Weight (kg) | Height (cm) | BMI (kg/m2) | Sequence |
|---|---|---|---|---|---|---|---|
| 1 | Female | 33 | 12 | 67.70 | 161 | 26.1 | B-A |
| 2 | Male | 22 | 6 | 72.85 | 175 | 23.8 | A-B |
| 3 | Male | 35 | 9 | 64.50 | 162 | 24.6 | A-B |
| 4 | Male | 34 | 13 | 62.45 | 176 | 20.2 | B-A |
| 5 | Female | 35 | 14 | 53.80 | 152 | 23.3 | A-B |
| 6 | Female | 32 | 12 | 69.80 | 166 | 25.3 | A-B |
| 7 | Female | 20 | 12 | 44.65 | 150 | 19.8 | B-A |
| 8 | Male | 35 | 12 | 72.25 | 164 | 26.9 | B-A |
| Average | 30.75 | 11.25 | 63.50 | 164 | 23.75 | ||
| Standard Deviation | 6.14 | 2.55 | 9.80 | 0.09 | 2.59 | ||
| %CV | 19.95 | 22.66 | 15.44 | 5.78 | 10.91 | ||
| Parameter | Intravenous | Intranasal |
|---|---|---|
| Cmax (ng/mL) | 136.15 (24.77) | 86.44 (18.5) |
| ke h−1 | 0.187 (0.02) | 0.183 (0.03) |
| t1/2 h | 3.75 (0.5) | 3.88 (0.6) |
| AUC 0-t (ng h/mL) | 490.8 (83.6) | 376.2 (97.0) |
| AUC 0-∞ (ng h/mL) | 548.2 (92) | 430.0 (106.2) |
| Tmax (h) | ||
| Median | 1.0 | |
| Minimum | 0.75 | |
| Maximum | 2.0 | |
| MRT (h) | 4.98 (0.64) | 5.8 (0.81) |
| Cl (L/h/) | 10.79 (1.91) | |
| Vd (L/) | 58.37 (11.9) |
| Parameter | Intravenous | Intranasal |
|---|---|---|
| Cmax (ng/mL) | 1982 (1125.7) | 873.55 (105.3) |
| ke h−1 | 0.33 (0.11) | 0.30 (0.03) |
| t 1/2 h | 2.2 (0.6) | 2.3 (0.25) |
| AUC 0-t (ng h/mL) | 4289.1 (778.1) | 4105.0 (1258.7) |
| AUC 0-∞ (ng h/mL) | 4435.1 (883.7) | 4232.0 (1242.29) |
| Tmax (h) | ||
| Median | 1.5 | |
| Minimum | 1.0 | |
| Maximum | 3.0 | |
| MRT (h) | 3.34 (0.73) | 4.5 (0.76) |
| Cl (L/h) | 14.52 (3.15) | |
| Vd (L) | 44.6 (7.28) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas, G.; Bobes, R.J.; Fragoso, G.; Pérez-Osorio, N.I.; Hernández, M.; Espinosa, A.; Fleury, A.; Flores, J.; The Revival Project Consortium; Laclette, J.P.; et al. Pharmacokinetic Study of Intranasal Dexamethasone and Methylprednisolone Compared with Intravenous Administration: Two Open-Label, Single-Dose, Two-Period, Two-Sequence, Cross-Over Study in Healthy Volunteers. Pharmaceutics 2023, 15, 105. https://doi.org/10.3390/pharmaceutics15010105
Cárdenas G, Bobes RJ, Fragoso G, Pérez-Osorio NI, Hernández M, Espinosa A, Fleury A, Flores J, The Revival Project Consortium, Laclette JP, et al. Pharmacokinetic Study of Intranasal Dexamethasone and Methylprednisolone Compared with Intravenous Administration: Two Open-Label, Single-Dose, Two-Period, Two-Sequence, Cross-Over Study in Healthy Volunteers. Pharmaceutics. 2023; 15(1):105. https://doi.org/10.3390/pharmaceutics15010105
Chicago/Turabian StyleCárdenas, Graciela, Raúl J. Bobes, Gladis Fragoso, Nicolas I. Pérez-Osorio, Marisela Hernández, Alejandro Espinosa, Agnes Fleury, José Flores, The Revival Project Consortium, Juan Pedro Laclette, and et al. 2023. "Pharmacokinetic Study of Intranasal Dexamethasone and Methylprednisolone Compared with Intravenous Administration: Two Open-Label, Single-Dose, Two-Period, Two-Sequence, Cross-Over Study in Healthy Volunteers" Pharmaceutics 15, no. 1: 105. https://doi.org/10.3390/pharmaceutics15010105