Self-Emulsifying Phospholipid Preconcentrates for the Enhanced Photoprotection of Luteolin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Assessment of Lut
2.3. Preparation of LSEPPs
2.4. Formulation Optimization
2.4.1. Droplet Size, Polydispersity Index (PDI), and Zeta-Potential
2.4.2. Dispersity Test
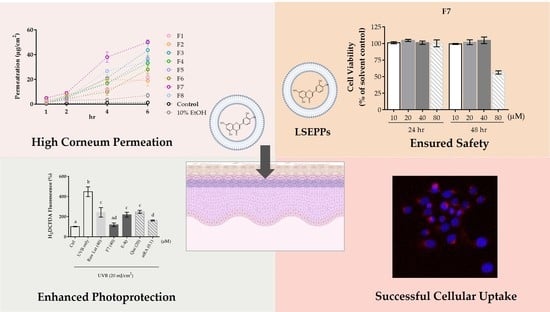
2.4.3. In Vitro Permeation Study
2.5. Characterization
2.5.1. Morphology
2.5.2. Compatibility between Lut and Excipients
2.5.3. Stability Evaluation
2.6. Cell Viability
2.6.1. Cell Culture
2.6.2. AlamarBlue® Assays
2.7. Cellular Uptake
2.8. Photoprotective Effects
2.8.1. Cell Viability after UVB Irradiation
2.8.2. Intracellular Reactive Oxygen Species Measurement
2.9. Statistics Analysis
3. Results
3.1. Solubility Assessment of Lut
3.2. Formulation Optimization
3.3. Physicochemical Characterization
3.4. Cell Viability
3.5. Cellular Uptake
3.6. Photoprotective Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rigel, D.S.; Lim, H.W.; Draelos, Z.D.; Weber, T.M.; Taylor, S.C. Photoprotection for all: Current gaps and opportunities. J. Am. Acad. Dermatol. 2022, 86, S18–S26. [Google Scholar] [CrossRef]
- Kaundinya, T.; Kundu, R.V.; Feinglass, J. The epidemiology of skin cancer by UV index: Cross-sectional analysis from the 2019 behavioral risk factor surveillance survey. Arch. Dermatol. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef]
- Souto, E.B.; Jäger, E.; Jäger, A.; Štěpánek, P.; Cano, A.; Viseras, C.; de Melo Barbosa, R.; Chorilli, M.; Zielińska, A.; Severino, P. Lipid Nanomaterials for Targeted Delivery of Dermocosmetic Ingredients: Advances in Photoprotection and Skin Anti-Aging. Nanomaterials 2022, 12, 377. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, J.; Yang, Z.; Xiong, L.; Li, L.; Gu, Z.; Li, Y. Polyphenolic sunscreens for photoprotection. Green Chem. 2022, 24, 3605–3622. [Google Scholar] [CrossRef]
- Pourzand, C.; Albieri-Borges, A.; Raczek, N.N. Shedding a New Light on Skin Aging, Iron-and Redox-Homeostasis and Emerging Natural Antioxidants. Antioxidants 2022, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-antioxidant activity relationships of luteolin and catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Miolo, G.; Innocenti, G.; Caffieri, S. The photodegradation of quercetin: Relation to oxidation. Molecules 2012, 17, 8898–8907. [Google Scholar] [CrossRef]
- Verschooten, L.; Smaers, K.; VanKelst, S.; Proby, C.; Maes, D.; Declercq, L.; Agostinis, P.; Garmyn, M. The flavonoid luteolin increases the resistance of normal, but not malignant keratinocytes, against UVB-induced apoptosis. J. Investig. Dermatol. 2010, 130, 2277–2285. [Google Scholar] [CrossRef]
- Yeager, D.G.; Lim, H.W. What’s new in photoprotection: A review of new concepts and controversies. Dermatol. Clin. 2019, 37, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Tammela, P.; Laitinen, L.; Galkin, A.; Wennberg, T.; Heczko, R.; Vuorela, H.; Slotte, J.P.; Vuorela, P. Permeability characteristics and membrane affinity of flavonoids and alkyl gallates in Caco-2 cells and in phospholipid vesicles. Arch. Biochem. Biophys. 2004, 425, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Choi, H.; Song, S.K.; Yu, J.W.; Lee, J.Y.; Choi, E.J.; Lee, D.H.; Do, S.H.; Kim, J.W. Nanoemulsion Vehicles as Carriers for Follicular Delivery of Luteolin. ACS Biomater. Sci. Eng. 2018, 4, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Alexander, A.; Saraf, S.; Saraf, S. Luteolin—Phospholipid complex: Preparation, characterization and biological evaluation. J. Pharm. Pharmacol. 2014, 66, 1451–1462. [Google Scholar] [CrossRef]
- Abidin, L.; Mujeeb, M.; Imam, S.S.; Aqil, M.; Khurana, D. Enhanced transdermal delivery of luteolin via non-ionic surfactant-based vesicle: Quality evaluation and anti-arthritic assessment. Drug Deliv. 2016, 23, 1079–1084. [Google Scholar] [CrossRef]
- Shehata, E.M.M.; Elnaggar, Y.S.R.; Galal, S.; Abdallah, O.Y. Self-emulsifying phospholipid pre-concentrates (SEPPs) for improved oral delivery of the anti-cancer genistein: Development, appraisal and ex-vivo intestinal permeation. Int. J. Pharm. 2016, 511, 745–756. [Google Scholar] [CrossRef]
- Dokania, S.; Joshi, A.K. Self-microemulsifying drug delivery system (SMEDDS)-challenges and road ahead. Drug Deliv. 2015, 22, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Van Staden, D.; du Plessis, J.; Viljoen, J. Development of topical/transdermal self-emulsifying drug delivery systems, not as simple as expected. Sci. Pharm. 2020, 88, 17. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK21054/ (accessed on 29 July 2022).
- Van Hoogevest, P.; Fahr, A. Phospholipids in Cosmetic Carriers. In Nanocosmetics; Cornier, J., Keck, C., Van de Voorde, M., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Yen, C.-C.; Chen, Y.-C.; Wu, M.-T.; Wang, C.-C.; Wu, Y.-T. Nanoemulsion as a strategy for improving the oral bioavailability and anti-inflammatory activity of andrographolide. Int. J. Nanomed. 2018, 13, 669–680. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R.; Shehata, E.M.M.; Galal, S.; Abdallah, O.Y. Self-emulsifying preconcentrates of daidzein-phospholipid complex: Design, in vitro and in vivo appraisal. Nanomedicine 2017, 12, 893–910. [Google Scholar] [CrossRef]
- Markovic, B.D.; Vladimirov, S.M.; Cudina, O.A.; Odovic, J.V.; Karljikovic-rajic, K.D. A PAMPA Assay as Fast Predictive Model of Passive Human Skin Permeability of New Synthesized Corticosteroid C-21 Esters. Molecules 2012, 17, 480–491. [Google Scholar] [CrossRef]
- Liu, W.-Y.; Hsieh, Y.-S.; Wu, Y.-T. Poly (Lactic-Co-Glycolic) Acid–Poly (Vinyl Pyrrolidone) Hybrid Nanoparticles to Improve the Efficiency of Oral Delivery of β-Carotene. Pharmaceutics 2022, 14, 637. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, T.; Saifullah, S.; Kawish, M.; Razzak, A.; Maharjan, R.; Imran, M.; Ali, I.; Roome, T.; Usman, S.; Raza, M. Design of absorption enhancer containing self-nanoemulsifying drug delivery system (SNEDDS) for curcumin improved anti-cancer activity and oral bioavailability. J. Mol. Liq. 2021, 324, 114774. [Google Scholar] [CrossRef]
- Jianxian, C.; Saleem, K.; Ijaz, M.; Ur-Rehman, M.; Murtaza, G.; Asim, M.H. Development and in vitro evaluation of gastro-protective aceclofenac-loaded self-emulsifying drug delivery system. Int. J. Nanomed. 2020, 15, 5217. [Google Scholar] [CrossRef]
- Fang, C.-L.; Wang, Y.; Tsai, K.H.-Y.; Chang, H.-I. Liposome-encapsulated baicalein suppressed lipogenesis and extracellular matrix formation in Hs68 human dermal fibroblasts. Front. Pharmacol. 2018, 9, 155. [Google Scholar] [CrossRef]
- Wu, H.-C.; Chen, Y.-F.; Cheng, M.-J.; Wu, M.-D.; Chen, Y.-L.; Chang, H.-S. Investigations into Chemical Components from Monascus purpureus with Photoprotective and Anti-Melanogenic Activities. J. Fungi 2021, 7, 619. [Google Scholar] [CrossRef] [PubMed]
- Naderi, R.; Pardakhty, A.; Abbasi, M.F.; Ranjbar, M. Preparation and evaluation of crocin loaded in nanoniosomes and their effects on ischemia—Reperfusion injuries in rat kidney. Sci. Rep. 2021, 11, 23525. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Dong, H.; Yang, X.; He, J.; Cai, S.; Xiao, K.; Zhu, L. Enhanced antioxidant activity, antibacterial activity and hypoglycemic effect of luteolin by complexation with manganese(II) and its inhibition kinetics on xanthine oxidase. RSC Adv. 2017, 7, 53385–53395. [Google Scholar] [CrossRef]
- Rajhard, S.; Hladnik, L.; Vicente, F.A.; Srčič, S.; Grilc, M.; Likozar, B. Solubility of luteolin and other polyphenolic compounds in water, nonpolar, polar aprotic and protic solvents by applying ftir/hplc. Processes 2021, 9, 1952. [Google Scholar] [CrossRef]
- Ajdary, M.; Keyhanfar, F.; Aflatoonian, R.; Amani, A.; Amjadi, F.S.; Zandieh, Z.; Mehdizadeh, M. Design and evaluation of a novel nanodrug delivery system for reducing the side effects of clomiphene citrate on endometrium. DARU J. Pharm. Sci. 2020, 28, 423–432. [Google Scholar] [CrossRef]
- Pereira, A.; Mallya, R. Formulation and evaluation of a photoprotectant cream containing Phyllanthus emblica extract- phospholipid complex. J. Pharmacogn. Phytochem. 2015, 4, 232–240. [Google Scholar]
- Hindarto, C.K.; Surini, S.; Permana, A.H.; Redjeki, S.; Irawan, C. Effect of mole ratio on physicochemical properties of luteolin-loaded phytosome Effect of mole ratio on physicochemical properties of luteolin-loaded phytosome. Pharma Innov. 2017, 6, 96–101. [Google Scholar]
- Perdue, J.D.; Seaton, P.J.; Tyrell, J.A.; Devido, D.R. The removal of Cremophor® EL from paclitaxel for quantitative analysis by HPLC–UV. J. Pharm. Biomed. Anal. 2006, 41, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Tănase, M.A.; Raducan, A.; Oancea, P.; Diţu, L.M.; Stan, M.; Petcu, C.; Scomoroşcenco, C.; Ninciuleanu, C.M.; Nistor, C.L.; Cinteza, L.O. Mixed pluronic—Cremophor polymeric micelles as nanocarriers for poorly soluble antibiotics—The influence on the antibacterial activity. Pharmaceutics 2021, 13, 435. [Google Scholar] [CrossRef]
- Ortiz-Tafoya, M.C.; Tecante, A. Physicochemical characterization of sodium stearoyl lactylate (SSL), polyoxyethylene sorbitan monolaurate (Tween 20) and κ-carrageenan. Data Brief 2018, 19, 642–650. [Google Scholar] [CrossRef]
- García, C.; Montero, G.; Coronado, M.A.; Valdez, B.; Stoytcheva, M.; Rosas, N.; Torres, R.; Sagaste, C.A. Valorization of Eucalyptus Leaves by Essential Oil Extraction as an Added Value Product in Mexico. Waste Biomass Valorization 2017, 8, 1187–1197. [Google Scholar] [CrossRef]
- Huang, M.; Su, E.; Zheng, F.; Tan, C. Encapsulation of flavonoids in liposomal delivery systems: The case of quercetin, kaempferol and luteolin. Food Funct. 2017, 8, 3198–3208. [Google Scholar] [CrossRef]
- Ferrari, R.; Lupi, M.; Colombo, C.; Morbidelli, M.; D’Incalci, M.; Moscatelli, D. Investigation of size, surface charge, PEGylation degree and concentration on the cellular uptake of polymer nanoparticles. Colloids Surf. B Biointerfaces 2014, 123, 639–647. [Google Scholar] [CrossRef]
- Van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef]
- Drescher, S.; van Hoogevest, P. The Phospholipid Research Center: Current Research in Phospholipids and Their Use in Drug Delivery. Pharmaceutics 2020, 12, 1235. [Google Scholar] [CrossRef] [PubMed]
- Subongkot, T.; Ngawhirunpat, T. Effect of liposomal fluidity on skin permeation of sodium fluorescein entrapped in liposomes. Int. J. Nanomed. 2015, 10, 4581–4592. [Google Scholar] [CrossRef] [Green Version]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Sinkó, B.; Garrigues, T.M.; Balogh, G.T.; Nagy, Z.K.; Tsinman, O.; Avdeef, A.; Takács-Novák, K. Skin–PAMPA: A new method for fast prediction of skin penetration. Eur. J. Pharm. Sci. 2012, 45, 698–707. [Google Scholar] [CrossRef]
- Zsikó, S.; Cutcher, K.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Baki, G.; Csányi, E.; Berkó, S. Nanostructured Lipid Carrier Gel for the Dermal Application of Lidocaine: Comparison of Skin Penetration Testing Methods. Pharmaceutics 2019, 11, 310. [Google Scholar] [CrossRef]
- Bae, I.H.; Park, J.W.; Kim, D.Y. Enhanced regenerative healing efficacy of a highly skin-permeable growth factor nanocomplex in a full-thickness excisional mouse wound model. Int. J. Nanomed. 2014, 9, 4551–4567. [Google Scholar] [CrossRef]
- Luo, L.; Patel, A.; Sinko, B.; Bell, M.; Wibawa, J.; Hadgraft, J.; Lane, M.E. A comparative study of the in vitro permeation of ibuprofen in mammalian skin, the PAMPA model and silicone membrane. Int. J. Pharm. 2016, 505, 14–19. [Google Scholar] [CrossRef]
- Vanti, G.; Grifoni, L.; Bergonzi, M.C.; Antiga, E.; Montefusco, F.; Caproni, M.; Bilia, A.R. Development and optimisation of biopharmaceutical properties of a new microemulgel of cannabidiol for locally-acting dermatological delivery. Int. J. Pharm. 2021, 607, 121036. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.W. Lipid-Protein-Partitioning theory of skin penetration enhancement. J. Control. Release 1991, 15, 237–248. [Google Scholar] [CrossRef]
- Fang, J.Y.; Tsai, T.H.; Lin, Y.Y.; Wong, W.W.; Wang, M.N.; Huang, J.F. Transdermal delivery of tea catechins and theophylline enhanced by terpenes: A mechanistic study. Biol. Pharm. Bull. 2007, 30, 343–349. [Google Scholar] [CrossRef]
- Nangare, S. Smart invasome synthesis, characterizations, pharmaceutical applications, and pharmacokinetic perspective: A review. Future J. Pharm. Sci. 2020, 8, 123. [Google Scholar] [CrossRef]
- Monti, D.; Chetoni, P.; Burgalassi, S.; Najarro, M.; Saettone, M.F.; Boldrini, E. Effect of different terpene-containing essential oils on permeation of estradiol through hairless mouse skin. Int. J. Pharm. 2002, 237, 209–214. [Google Scholar] [CrossRef]
- Southwell, I.A.; Freeman, S.; Rubel, D. Skin Irritancy of Tea Tree Oil. J. Essent. Oil Res. 1997, 9, 47–52. [Google Scholar] [CrossRef]
- Lee, J.; Ha, S.J.; Park, J.; Kim, Y.H.; Lee, N.H.; Kim, Y.E.; Kim, Y.; Song, K.-M.; Jung, S.K. 1,8-cineole prevents UVB-induced skin carcinogenesis by targeting the aryl hydrocarbon receptor. Oncotarget 2017, 8, 105995. [Google Scholar] [CrossRef]
- Safta, D.A.; Bogdan, C.; Moldovan, M.L. Vesicular Nanocarriers for Phytocompounds in Wound Care: Preparation and Characterization. Pharmaceutics 2022, 14, 991. [Google Scholar] [CrossRef]
- Lima, A.C.; Reis, R.L.; Ferreira, H.; Neves, N.M. Cellular uptake of three different nanoparticles in an inflammatory arthritis scenario versus normal conditions. Mol. Pharm. 2021, 18, 3235–3246. [Google Scholar] [CrossRef] [PubMed]
- Skóra, B.; Piechowiak, T.; Szychowski, K.A.; Gmiński, J. Entrapment of silver nanoparticles in L-α-phosphatidylcholine/cholesterol-based liposomes mitigates the oxidative stress in human keratinocyte (HaCaT) cells. Eur. J. Pharm. Biopharm. 2021, 166, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Dixit, V.K. Development and evaluation of vesicular system for curcumin delivery. Arch. Dermatol. Res. 2011, 303, 89–101. [Google Scholar] [CrossRef]









| Formulation | PHOSAL® 50 PG | Kolliphor® EL | Tween® 20 | 1,8-cineole | Nerolidol |
|---|---|---|---|---|---|
| Wt% | |||||
| F1 | 20 | 70 | 10 | - | - |
| F2 | 20 | 65 | 15 | - | - |
| F3 | 25 | 65 | 10 | - | - |
| F4 | 25 | 60 | 15 | - | - |
| F5 | 30 | 60 | 10 | - | - |
| F6 | 30 | 55 | 15 | - | - |
| F7 | 28.5 | 57 | 9.5 | 5 | - |
| F8 | 28.5 | 57 | 9.5 | - | 5 |
| Formulation | Size (nm) | PDI 1 | Zeta-Potential (mV) | Dispersity Grade |
|---|---|---|---|---|
| F1 | 973.1 ± 46.1 a | 0.586 ± 0.017 a | −10.2 ± 0.5 a | B |
| F2 | 1055.9 ± 181.7 ab | 0.515 ± 0.020 ab | −10.9 ± 0.9 a | B |
| F3 | 1237.6 ± 202.6 a | 0.441 ± 0.040 b | −11.3 ± 1.2 a | B |
| F4 | 893.0 ± 81.5 a | 0.574 ± 0.111 a | −12.2 ± 0.4 ab | B |
| F5 | 253.7 ± 6.3 cd | 0.375 ± 0.038 c | −11.9 ± 0.9 ab | C |
| F6 | 459.0 ± 37.3 c | 0.426 ± 0.015 c | −11.3 ± 0.7 ab | C |
| F7 | 140.6 ± 24.2 d | 0.318 ± 0.015 c | −12.1 ± 1.6 a | C |
| F8 | 164.9 ± 13.9 d | 0.317 ± 0.013 c | −13.4 ± 2.2 b | C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, Y.-S.; Chen, Y.-F.; Cheng, Y.-Y.; Liu, W.-Y.; Wu, Y.-T. Self-Emulsifying Phospholipid Preconcentrates for the Enhanced Photoprotection of Luteolin. Pharmaceutics 2022, 14, 1896. https://doi.org/10.3390/pharmaceutics14091896
Hsieh Y-S, Chen Y-F, Cheng Y-Y, Liu W-Y, Wu Y-T. Self-Emulsifying Phospholipid Preconcentrates for the Enhanced Photoprotection of Luteolin. Pharmaceutics. 2022; 14(9):1896. https://doi.org/10.3390/pharmaceutics14091896
Chicago/Turabian StyleHsieh, Yun-Shan, Yih-Fung Chen, Yung-Yi Cheng, Wan-Yi Liu, and Yu-Tse Wu. 2022. "Self-Emulsifying Phospholipid Preconcentrates for the Enhanced Photoprotection of Luteolin" Pharmaceutics 14, no. 9: 1896. https://doi.org/10.3390/pharmaceutics14091896