Nanoscale Delivery Systems of Lutein: An Updated Review from a Pharmaceutical Perspective
Abstract
:1. Introduction
2. Overview of Lutein
2.1. Structure and Biological Behavior of Lutein
2.2. Therapeutic Potential of Lutein
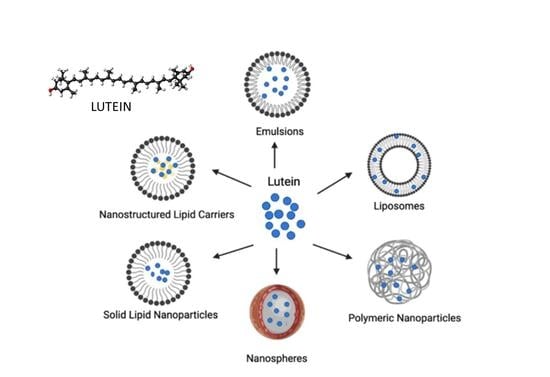
3. Nanoscale Delivery Systems of Lutein
3.1. Liposomes
3.2. Emulsion-Based Systems
3.3. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
3.4. Polymer-Based Nanoparticles
3.5. Polymer/Lipid-Based Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moosavi, M.A.; Haghi, A.; Rahmati, M.; Taniguchi, H.; Mocan, A.; Echeverria, J.; Gupta, V.K.; Tzvetkov, N.T.; Atanasov, A.G. Phytochemicals as potent modulators of autophagy for cancer therapy. Cancer Lett. 2018, 424, 46–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134 (Suppl. S12), 3479–3485. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary sources, extraction, encapsulation, bioavailability, and health benefits—A review of recent advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How effective are they to prevent age-related diseases? Molecules 2019, 24, 1801. [Google Scholar]
- Maiani, G.; Caston, M.J.; Catasta, G.; Toti, E.; Cambrodon, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar]
- Namitha, K.K.; Negi, P.S. Chemistry and biotechnology of carotenoids. Crit. Rev. Food Sci. Nutr. 2010, 50, 728–760. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar]
- Cazzaniga, S.; Bressan, M.; Carbonera, D.; Agostini, A.; Dall’Osto, L. Diferential roles of carotenes and xanthophylls in photosystem I photoprotection. Biochemistry 2016, 55, 3636–3649. [Google Scholar]
- Alves-Rodrigues, A.; Shao, A. The science behind lutein. Toxicol. Lett. 2004, 150, 57–83. [Google Scholar]
- Park, H.-A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-apoptotic effects of carotenoids in neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef]
- Snodderly, D.M.; Auran, J.D.; Delori, F.C. The macular pigment. II. Spatial distribution in primate retinas. Investig. Ophthalmol. Vis. Sci. 1984, 25, 674–685. [Google Scholar]
- Arunkumar, R.; Calvo, C.M.; Conrady, C.D.; Bernstein, P.S. What do we know about the macular pigment in AMD: The past, the present, and the future. Eye 2018, 32, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A mechanistic review of β-carotene, lutein, and zeaxanthin in eye health and disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef]
- Al Mijan, M.; Sim, W.-J.; Lim, T.-G. Physiological Effects of Green-Colored Food-Derived Bioactive Compounds on Cancer. Appl. Sci. 2021, 11, 11288. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.-S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169–1185. [Google Scholar]
- Roberts, R.L.; Green, J.; Lewis, B. Lutein and zeaxanthin in eye and skin health. Clin. Dermatol. 2009, 27, 195–201. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; López-Pozo, M.; Stewart, J.J.; Adams, W.W., III. Zeaxanthin and lutein: Photoprotectors, anti-inflammatories, and brain food. Molecules 2020, 25, 3607. [Google Scholar] [CrossRef]
- Hammond, B.R., Jr.; Wooten, B.R.; Snodderly, D.M. Individual variations in the spatial profile of human macular pigment. J. Opt. Soc. Am. B 1997, 14, 1187–1196. [Google Scholar] [CrossRef]
- Subczynski, W.; Wisniewska, A.; Widomska, J. Location of macular xanthophylls in the most vulnerable regions of photoreceptor outer-segment membranes. Arch. Biochem. Biophys. 2010, 504, 61–66. [Google Scholar] [CrossRef]
- Lem, D.W.; Davey, P.G.; Gierhart, D.L.; Rosen, R.B. A systematic review of carotenoids in the management of age-related macular degeneration. Antioxidants 2021, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and zeaxanthin and their roles in age-related macular degeneration—Neurodegenerative disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef] [PubMed]
- Lem, D.W.; Gierhart, D.L.; Davey, P.G. A systematic review of carotenoids in the management of diabetic retinopathy. Nutrients 2021, 13, 2441. [Google Scholar] [CrossRef]
- Manayi, A.; Abdollahi, M.; Raman, T.; Nabavi, S.F.; Habtemariam, S.; Daglia, M.; Nabavi, S.M. Lutein and cataract: From bench to bedside. Crit. Rev. Biotechnol. 2016, 36, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Shegokar, R.; Mitri, K. Carotenoid lutein: A promising candidate for pharmaceutical and nutraceutical applications. J. Diet. Suppl. 2012, 9, 183–210. [Google Scholar] [CrossRef] [PubMed]
- Madaan, T.; Choudhary, A.N.; Gyenwalee, S.; Thomas, S.; Mishra, H.; Tariq, M.; Vohora, D.; Talegaonkar, S. Lutein, a versatile phyto-nutraceutical: An insight on pharmacology, therapeutic indications, challenges and recent advances in drug delivery. Pharma Nutr. 2017, 5, 64–75. [Google Scholar] [CrossRef]
- De Felice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar]
- Santini, A.; Novellino, E. To Nutraceuticals and Back: Rethinking a Concept. Foods 2017, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Santini, A.; Novellino, E. Nutraceuticals: Beyond the Diet before the Drugs. Curr. Bioact. Compd. 2014, 10, 1–12. [Google Scholar] [CrossRef]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals in hypercholesterolaemia: An Overview. Br. J. Pharmacol. 2017, 174, 1450–1463. [Google Scholar] [PubMed]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2019, 12, 1–7. [Google Scholar] [PubMed]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [PubMed]
- Santini, A.; Novellino, E. Nutraceuticals: Shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, R.; Gorusupudi, A.; Bernstein, P.S. The macular carotenoids: A biochemical overview. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158617. [Google Scholar] [CrossRef]
- Mitra, S.; Rauf, A.; Tareq, A.M.; Jahan, S.; Emran, T.B.; Shahriar, T.G.; Dhama, K.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Rebezov, M.; et al. Potential health benefits of carotenoid lutein: An updated review. Food Chem. Toxicol. 2021, 154, 112328. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Lee, J.C.; Leung, H.H.; Lam, W.C.; Fu, Z.; Lo, A.C.Y. Lutein supplementation for eye diseases. Nutrients 2020, 12, 1721. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.-H.; Nam, H.-Y.; Lew, S.-Y.; Naidu, M.; David, P.; Kamalden, T.A.; Hadie, S.N.H.; Lim, L.-W. Discovering the Potential of Natural Antioxidants in Age-Related Macular Degeneration: A Review. Pharmaceuticals 2022, 15, 101. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoscale nutrient delivery systems for food applications: Improving bioactive dispersibility, stability, and bioavailability. J. Food Sci. 2015, 80, 1602–1611. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, L.; Tan, L. Effectiveness of nanoscale delivery systems on improving the bioavailability of lutein in rodent models: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 62, 2375–2390. [Google Scholar] [CrossRef]
- Conn, P.F.; Schalch, W.; Truscott, T.G. The singlet oxygen and carotenoid interaction. J. Photochem. Photobiol. B 1991, 11, 41–47. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Polutchko, S.K.; Adams, W.W., III. Structure-function-environment relationship of the isomers zeaxanthin and lutein. Photochem 2022, 2, 308–325. [Google Scholar] [CrossRef]
- Becerra, M.O.; Contreras, L.M.; Lo, M.H.; Diaz, J.M.; Herrera, G.C. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Widomska, J.; Zareba, M.; Subczynski, W.K. Can xanthophyll-membrane interactions explain their selective presence in the retina and brain? Foods 2016, 5, 7. [Google Scholar]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar]
- Ahn, Y.J.; Kim, H. Lutein as a modulator of oxidative stress-mediated inflammatory diseases. Antioxidants 2021, 10, 1448. [Google Scholar]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T. Lutein: More than just a filter for blue light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef]
- Sujak, A.; Gabrielska, J.; Grudzinski, W.; Borc, R.; Mazurek, P.; Gruszecki, W.I. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: The structural aspects. Arch. Biochem. Biophys. 1999, 371, 301–307. [Google Scholar] [CrossRef]
- Gabrielska, J.; Gruszecki, W.I. Zeaxanthin (dihydroxy-β-carotene) but not β-carotene rigidifies lipid membranes: A 1H-NMR study of carotenoid-egg phosphatidylcholine liposomes. Biochim. Biophys. Acta 1996, 1285, 167–174. [Google Scholar]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta 2005, 1740, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Grudzinski, W.; Nierzwicki, L.; Welc, R.; Reszczynska, E.; Luchowski, R.; Czub, J.; Gruszecki, W.I. Localization and orientation of xanthophylls in a lipid bilayer. Sci. Rep. 2017, 7, 9619. [Google Scholar] [CrossRef] [PubMed]
- Widomska, J.; Subczynski, W.K. Why has nature chosen lutein and zeaxanthin to protect the retina? J. Clin. Exp. Ophthalmol. 2014, 5, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranard, K.M.; Jeon, S.; Mohn, E.S.; Griffiths, J.C.; Johnson, E.J.; Erdman, J.W., Jr. Dietary guidance for lutein: Consideration for intake recommendations is scientifically supported. Eur. J. Nutr. 2017, 56, 37–42. [Google Scholar] [PubMed]
- Sauer, L.; Li, B.; Bernstein, P.S. Ocular carotenoid status in health and disease. Annu. Rev. Nutr. 2019, 39, 95–120. [Google Scholar]
- Sommerburg, O.; Keunen, J.E.E.; Bird, A.C.; Van Kuijk, F.J.G.M. Fruits and vegetables that are sources for lutein and zeaxanthin: The macular pigment in human eyes. Br. J. Ophthalmol. 1998, 82, 907–910. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Doraiswamy, S.; Harrison, E.H. Xanthophylls are preferentially taken up compared with β-carotene by retinal cells via a SRBI-dependent mechanism. J. Lipid Res. 2008, 49, 1715–1724. [Google Scholar] [PubMed]
- Yoo, J.H.; Shanmugam, S.; Thapa, P.; Lee, E.S.; Balakrishnan, P.; Baskaran, R.; Yoon, S.K.; Choi, H.G.; Yong, C.S.; Yoo, B.K.; et al. Novel self-nanoemulsifying drug delivery system for enhanced solubility and dissolution of lutein. Arch. Pharm. Res. 2010, 33, 417–426. [Google Scholar] [PubMed]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The effect of lutein on eye and extra-eye health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef]
- Weigert, G.; Kaya, S.; Pemp, B.; Sacu, S.; Lasta, M.; Werkmeister, R.M.; Dragostinoff, N.; Simader, C.; Garhöfer, G.; Schmidt-Erfurth, U.; et al. Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8174–8178. [Google Scholar]
- Koushan, K.; Rusovici, R.; Li, W.; Ferguson, L.R.; Chalam, K.V. The role of lutein in eye-related disease. Nutrients 2013, 5, 1823–1839. [Google Scholar]
- Richer, S.; Stiles, W.; Statkute, L.; Pulido, J.; Frankowski, J.; Rudy, D.; Pei, K.; Tsipursky, M.; Nyland, J. Double-masked placebo controlled randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004, 75, 216–229. [Google Scholar] [CrossRef]
- Bian, Q.; Qin, T.; Ren, Z.; Wu, D.; Shang, F. Lutein or zeaxanthin supplementation suppresses inflammatory responses in retinal pigment epithelial cells and macrophages. Adv. Exp. Med. Biol. 2012, 723, 43–50. [Google Scholar] [PubMed]
- Kamoshita, M.; Toda, E.; Osada, H.; Narimatsu, T.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci. Rep. 2016, 6, 30226. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, W.; Zhou, X.; Long, C.; Kuang, X.; Hu, J.; Tang, Y.; Liu, L.; He, J.; Huang, Z.; et al. Protective effect of lutein on ARPE-19 cells upon H2O2-induced G2/M arrest. Mol. Med. Rep. 2017, 16, 2069–2074. [Google Scholar] [CrossRef]
- Padmanabha, S.; Vallikannan, B. Fatty acids modulate the efficacy of lutein in cataract prevention: Assessment of oxidative and inflammatory parameters in rats. Biochem. Biophys. Res. Commun. 2018, 500, 435–442. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Landrum, J.I.; Bone, R.A. Biological mechanisms of the protective role of lutein and zeaxanthin in the eye. Ann. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef]
- Fuad, N.I.N.; Sekar, M.; Gan, S.H.; Lum, P.T.; Vaijanathappa, J.; Ravi, S. Lutein: A comprehensive review on its chemical, biological activities and therapeutic potentials. Pharmacogn. J. 2020, 12, 1769–1778. [Google Scholar] [CrossRef]
- Park, J.S.; Chew, B.P.; Wong, T.S. Dietary lutein from marigold extract inhibits mammary tumor development in BALB/c mice. J. Nutr. 1998, 128, 1650–1656. [Google Scholar] [CrossRef]
- Chew, B.P.; Brown, C.M.; Park, J.S.; Mixter, P.F. Dietary lutein inhibits mouse mammary tumor growth by regulating angiogenesis and apoptosis. Anticancer Res. 2003, 23, 3333–3339. [Google Scholar]
- Sindhu, E.R.; Firdous, A.P.; Ramnath, V.; Kuttan, R. Effect of carotenoid lutein on N-nitrosodiethylamine-induced hepatocellular carcinoma and its mechanism of action. Eur. J. Cancer Prev. 2013, 22, 320–327. [Google Scholar] [CrossRef]
- Gong, X.; Smith, J.; Swanson, H.; Rubin, L. Carotenoid Lutein Selectively Inhibits Breast Cancer Cell Growth and Potentiates the Effect of Chemotherapeutic Agents through ROS-Mediated Mechanisms. Molecules 2018, 23, 905. [Google Scholar] [CrossRef] [PubMed]
- Rafi, M.M.; Kanakasabai, S.; Gokarn, S.V.; Krueger, E.G.; Bright, J.J. Dietary lutein modulates growth and survival genes in prostate cancer cells. J. Med. Food 2015, 18, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Liu, X.; Wang, M.; Wang, P.; Yang, J.; Zhang, S. Lutein inhibits proliferation, invasion and migration of hypoxic breast cancer cells via downregulation of HES1. Int. J. Oncol. 2018, 52, 2119–2129. [Google Scholar] [CrossRef]
- Zhang, W.-L.; Zhao, Y.-N.; Shi, Z.-Z.; Cong, D.; Bai, Y.-S. Lutein inhibits cell growth and activates apoptosis via the PI3K/AKT/mTOR signaling pathway in A549 human non-small-cell lung cancer cells. J. Environ. Pathol. Toxicol. Oncol. Off. Organ. Int. Soc. Environ. Toxicol. Cancer 2018, 37, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Gansukh, E.; Mya, K.K.; Jung, M.; Keum, Y.S.; Kim, D.H.; Saini, R.K. Lutein derived from marigold (Tagetes erecta) petals triggers ROS generation and activates Bax and caspase-3 mediated apoptosis of human cervical carcinoma (HeLa) cells. Food Chem. Toxicol. 2019, 127, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kavalappa, Y.P.; Gopal, S.S.; Ponesakki, G. Lutein inhibits breast cancer cell growth by suppressing antioxidant and cell survival signals and induces apoptosis. J. Cell. Physiol. 2021, 236, 1798–1809. [Google Scholar] [CrossRef]
- Shin, J.; Song, M.-H.; Oh, J.-W.; Keum, Y.-S.; Saini, R.K. Pro-oxidant actions of carotenoids in triggering apoptosis of cancer cells: A review of emerging evidence. Antioxidants 2020, 9, 532. [Google Scholar] [CrossRef]
- Mecocci, P.; Polidori, M.C.; Cherubini, A.; Ingegni, T.; Mattioli, P.; Catani, M.; Rinaldi, P.; Cecchetti, R.; Stahl, W.; Senin, U.; et al. Lymphocyte oxidative DNA damage and plasma antioxidants in Alzheimer disease. Arch. Neurol. 2002, 59, 794–798. [Google Scholar] [CrossRef]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef]
- Min, J.Y.; Min, K.B. Serum lycopene, lutein and zeaxanthin, and the risk of Alzheimer’s disease mortality in older adults. Dement. Geriatr. Cogn. Disord. 2014, 37, 246–256. [Google Scholar] [CrossRef]
- Feeney, J.; O’Leary, N.; Moran, R.; O’Halloran, A.M.; Nolan, J.M.; Beatty, S.; Young, I.S.; Kenny, R.A. Plasma lutein and zeaxanthin are associated with better cognitive function across multiple domains in a large population-based sample of older adults: Findings from the Irish longitudinal study on aging. J. Gerontol. A 2017, 72, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Yu, X.; Chen, M.; Chen, J.; Xu, J. Lutein protects against severe traumatic brain injury through anti-inflammation and antioxidative effects via ICAM-1/Nrf-2. Mol. Med. Rep. 2017, 16, 4235–4240. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, Y.; Wu, Y.; Zhang, Y.; Wang, Z.; Liu, X. Lutein suppresses inflammatory responses through Nrf2 activation and NF-κB inactivation in lipopolysaccharide-stimulated BV-2 microglia. Mol. Nutr. Food Res. 2015, 59, 1663–1673. [Google Scholar] [CrossRef]
- Shimazu, Y.; Kobayashi, A.; Endo, S.; Takemura, J.; Takeda, M. Effect of lutein on the acute inflammation-induced c-Fos expression of rat trigeminal spinal nucleus caudalis and C1 dorsal horn neurons. Eur. J. Oral. Sci. 2019, 127, 379–385. [Google Scholar] [CrossRef]
- Stringham, J.M.; Johnson, E.J.; Hammond, B.R. Lutein across the lifespan: From childhood cognitive performance to the aging eye and brain. Curr. Dev. Nutr. 2019, 3, nzz066. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.H.; Navab, M.; Dwyer, K.M.; Hassan, K.; Sun, P.; Shircore, A.; Hama-Levy, S.; Hough, G.; Wang, X.; Drake, T.; et al. Oxygenated carotenoid lutein and progression of early atherosclerosis: The Los Angeles atherosclerosis study. Circulation 2001, 103, 2922–2927. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Xu, X.; Huang, Y.; Xiao, X.; Ma, L.; Sun, T.; Dong, P.; Wang, X.; Lin, X. High serum level of lutein may be protective against early atherosclerosis: The Beijing atherosclerosis study. Atherosclerosis 2011, 219, 789–793. [Google Scholar] [CrossRef]
- Chung, R.W.S.; Leanderson, P.; Lundberg, A.K.; Jonasson, L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 2017, 262, 87–93. [Google Scholar] [CrossRef]
- Lidebjer, C.; Leanderson, P.; Ernerudh, J.; Jonasson, L. Low plasma levels of oxygenated carotenoids in patients with coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 448–456. [Google Scholar] [CrossRef]
- Howard, A.N.; Thurnham, D.I. Lutein and atherosclerosis: Belfast versus Toulouse revisited. Med. Hypotheses 2017, 98, 63–68. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Selvaraj, R.K. Dietary lutein and fish oil interact to alter atherosclerotic lesions in a Japanese quail model of atherosclerosis. J. Anim. Physiol. Anim. Nutr. 2011, 95, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Gillespie, C.; Ballew, C.; Sowell, A.; Mannino, D. Serum carotenoid concentrations in US children and adolescents. Am. J. Clin. Nutr. 2002, 76, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.S.; Eligar, S.M.; Vallikannan, B.; Ponesakki, G. Inhibitory efficacy of lutein on adipogenesis is associated with blockage of early phase regulators of adipocyte differentiation. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2021, 1866, 158812. [Google Scholar] [CrossRef]
- Ford, E.S.; Mokdad, A.H.; Giles, W.H.; Brown, D.W. The metabolic syndrome and antioxidant concentrations: Findings from the Third National Health and Nutrition Examination Survey. Diabetes 2003, 52, 2346–2352. [Google Scholar] [CrossRef] [PubMed]
- Sluijs, I.; Beulens, J.W.; Grobbee, D.E.; van der Schouw, Y.T. Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. J. Nutr. 2009, 139, 987–992. [Google Scholar] [CrossRef]
- Tuzcu, M.; Orhan, C.; Muz, O.E.; Sahin, N.; Juturu, V.; Sahin, K. Lutein and zeaxanthin isomers modulates lipid metabolism and the inflammatory state of retina in obesity-induced high-fat diet rodent model. BMC Ophthalmol. 2017, 17, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, E.; Quadro, L. Lutein, zeaxanthin and mammalian development: Metabolism, functions and implications for health. Arch. Biochem. Biophys. 2018, 647, 33–40. [Google Scholar] [CrossRef]
- Ozawa, Y.; Sasaki, M.; Takahashi, N.; Kamoshita, M.; Miyake, S.; Tsubota, K. Neuroprotective effects of lutein in the retina. Curr. Pharm. Des. 2012, 18, 51–56. [Google Scholar] [CrossRef]
- Ribaya-Mercado, J.D.; Blumberg, J.B. Lutein and zeaxanthin and their potential roles in disease prevention. J. Am. Coll. Nutr. 2004, 23, 567S–587S. [Google Scholar] [CrossRef]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Noda, K.; Imamura, Y.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neuroprotective effect of an antioxidant, lutein, during retinal inflammation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1433–1439. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Zhao, R.; Wang, D.; Ma, Y.; Ai, L. Plant natural products: Promising resources for cancer chemoprevention. Molecules 2021, 26, 933. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-P.; Sun, L.; Yu, H.-S.; Liang, L.-P.; Li, W.; Ding, H.; Song, X.-B.; Zhang, L.-J. The pharmacological effects of lutein and zeaxanthin on visual disorders and cognition diseases. Molecules 2017, 22, 610. [Google Scholar] [CrossRef]
- Liu, T.; Liu, W.H.; Zhao, J.S.; Meng, F.Z.; Wang, H. Lutein protects against β-amyloid peptide-induced oxidative stress in cerebrovascular endothelial cells through modulation of Nrf-2 and NF-κb. Cell. Biol. Toxicol. 2017, 33, 57–67. [Google Scholar] [CrossRef]
- Li, S.; Ding, Y.; Niu, Q.; Xu, S.; Pang, L.; Ma, R.; Jing, M.; Feng, G.; Tang, J.X.; Zhang, Q.; et al. Lutein has a protective effect on hepatotoxicity induced by arsenic via Nrf2 signaling. BioMed Res. Int. 2015, 2015, 315205. [Google Scholar] [CrossRef]
- Rafi, M.M.; Shafaie, Y. Dietary lutein modulates inducible nitric oxide synthase (iNOS) gene and protein expression in mouse macrophage cells (RAW 264.7). Mol. Nutr. Food Res. 2007, 51, 333–340. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, D.; Hong, Y.M.; Mizuno, S.; Joo, C.K. Inhibition of nNOS and COX-2 expression by lutein in acute retinal ischemia. Nutrition 2006, 22, 668–671. [Google Scholar] [CrossRef]
- Du, S.Y.; Zhang, Y.L.; Bai, R.X.; Ai, Z.L.; Xie, B.S.; Yang, H.Y. Lutein prevents alcohol-induced liver disease in rats by modulating oxidative stress and inflammation. Int. J. Clin. Exp. Med. 2015, 8, 8785–8793. [Google Scholar]
- Kim, J.E.; Clark, R.M.; Park, Y.; Lee, J.; Fernandez, M.L. Lutein decreases oxidative stress and inflammation in liver and eyes ofguinea pigs fed a hypercholesterolemic diet. Nutr. Res. Pract. 2012, 6, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Muriach, M.; Bosch-Morell, F.; Arnal, E.; Alexander, G.; Blomhoff, R.; Romero, F.J. Lutein prevents the effect of high glucose levels on immune system cells in vivo and in vitro. J. Physiol. Biochem. 2008, 64, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Na, H.-J.; Kim, C.-K.; Kim, J.-Y.; Ha, K.-S.; Lee, H.; Chung, H.-T.; Kwon, H.J.; Kwon, Y.-G.; Kim, Y.-M. The non-provitamin A carotenoid, lutein, inhibits NF-κB-dependent gene expression through redox-based regulation of the phosphatidylinositol 3-kinase/PTEN/Akt and NF-κB-inducing kinase pathways: Role of H2O2 in NF-κB activation. Free Radic. Biol. Med. 2008, 45, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhang, Y.; Li, Y.; Lu, K.; Shen, Y.; Guo, Y.; Qi, Q.; Wang, M.; Zhang, S. NrF2/ARE and NF-κB pathway regulation may be the mechanism for lutein inhibition of human breast cancer cell. Future Oncol. 2018, 14, 719–726. [Google Scholar] [CrossRef]
- Ouyang, B.; Li, Z.; Ji, X.; Huang, J.; Zhang, H.; Jiang, C. The protective role of lutein on isoproterenol-induced cardiac failure rat model through improving cardiac morphology, antioxidant status via positively regulating Nrf2/HO-1 signalling pathway. Pharm. Biol. 2019, 57, 529–535. [Google Scholar] [CrossRef]
- Qiao, Y.Q.; Jiang, P.F.; Gao, Y.Z. Lutein prevents osteoarthritis through Nrf2 activation and downregulation of inflammation. Arch. Med. Sci. 2018, 14, 617–624. [Google Scholar] [CrossRef]
- Zhao, C.; Shen, X.; Guo, M. Stability of lutein encapsulated whey protein nano-emulsion during storage. PLoS ONE 2018, 13, e0192511. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. Recent Advances on Nanoparticle Based Strategies for Improving Carotenoid Stability and Biological Activity. Antioxidants 2021, 10, 713. [Google Scholar] [CrossRef]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part I—Clinical Trials Legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU. Pharmaceutics 2020, 12, 146. [Google Scholar] [CrossRef]
- Durazzo, A.; Nazhand, A.; Lucarini, M.; Atanasov, A.G.; Souto, E.B.; Novellino, E.; Capasso, R.; Santini, A. An Updated Overview on Nanonutraceuticals: Focus on Nanoprebiotics and Nanoprobiotics. Int. J. Mol. Sci. 2020, 21, 2285. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Souto, E.B.; Durazzo, A.; Lucarini, M.; Novellino, E.; Tewari, D.; Wang, D.; Atanasov, A.G.; Santini, A. Big impact of nanoparticles: Analysis of the most cited nanopharmaceuticals and nanonutraceuticals research. Curr. Res. Biotechnol. 2020, 2, 53–63. [Google Scholar] [CrossRef]
- Zhao, C.D.; Cheng, H.; Jiang, P.F.; Yao, Y.J.; Han, J. Preparation of lutein loaded particles for improving solubility and stability by Polyvinylpyrrolidone (PVP) as an emulsion-stabilizer. Food Chem. 2014, 156, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Nutraceutical nanodelivery; An Insight into the bioaccessibility/bioavailability of different bioactive compounds loaded within nanocarriers. Crit. Rev. Food Sci. Nutr. 2020, 61, 3031–3065. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioactive-loaded nanocarriers for functional foods: From designing to bioavailability. Curr. Opin. Food Sci. 2020, 33, 21–29. [Google Scholar] [CrossRef]
- Leitgeb, M.; Knez, Ž.; Primožič, M. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984–105001. [Google Scholar]
- Sarangi, M.; Padhi, S. Novel herbal drug delivery system: An overview. Arch. Med. Health Sci. 2018, 6, 171. [Google Scholar] [CrossRef]
- Esposto, B.S.; Jauregi, P.; Tapia-Blácido, D.R.; Martelli-Tosi, M. Liposomes vs. chitosomes: Encapsulating food bioactives. Trends Food Sci. Technol. 2021, 108, 40–48. [Google Scholar] [CrossRef]
- Sogut, O.; Aydemir Sezer, U.; Sezer, S. Liposomal delivery systems for herbal extracts. J. Drug Deliv. Sci. Technol. 2021, 61, 102147. [Google Scholar] [CrossRef]
- Ulrich, A.S. Biophysical aspects of using liposomes as delivery vehicles. Biosci. Rep. 2002, 22, 129–150. [Google Scholar] [CrossRef]
- Tan, C.; Xia, S.; Xue, J.; Xie, J.; Feng, B.; Zhang, X. Liposomes as Vehicles for Lutein: Preparation, Stability, Liposomal Membrane Dynamics, and Structure. J. Agric. Food Chem. 2013, 61, 8175–8184. [Google Scholar] [CrossRef]
- Tan, C.; Xue, J.S.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Liposomes as a delivery system for carotenoids: Comparative antioxidant activity of carotenoids as measured by ferric reducing antioxidant power, DPPH assay and lipid peroxidation. J. Agric. Food Chem. 2014, 62, 6726–6735. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Xue, J.; Lou, X.W.; Abbas, S.; Guan, Y.; Feng, B.; Zhang, X.M.; Xia, S.Q. Liposomes as delivery systems for carotenoids: Comparative studies of loading ability, storage stability and in vitro release. Food Funct. 2014, 5, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zhang, Y.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Modulation of the carotenoid bioaccessibility through liposomal encapsulation. Colloids Surf. B Biointerfaces 2014, 123, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Tan, C.; Zhang, Y.; Abbas, S.; Feng, B.; Zhang, X.; Qin, F. Modulating effect of lipid bilayer–carotenoid interactions on the property of liposome encapsulation. Colloids Surf. B Biointerfaces 2015, 128, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Hu, D.; Jin, H.; Zhao, Y.; Liang, J. Preparation of lutein proliposomes by supercritical anti-solvent technique. Food Hydrocoll. 2012, 26, 456–463. [Google Scholar] [CrossRef]
- Zhao, L.S.; Temelli, F.; Curtis, J.M.; Chen, L.Y. Encapsulation of lutein in liposomes using supercritical carbon dioxide. Food Res. Int. 2017, 100, 168–179. [Google Scholar] [CrossRef]
- Trucillo, P.; Martino, M.; Reverchon, E. Supercritical Assisted Production of Lutein-Loaded Liposomes and Modelling of Drug Release. Processes 2021, 9, 1162. [Google Scholar] [CrossRef]
- Tan, C.; Feng, B.; Zhang, X.; Xia, W.; Xia, S. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocoll. 2016, 52, 774–784. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, D.; Liu, C.; Chang, Y.; Song, J.; Xiao, Y.J.R.A. Polypeptide–decorated nanoliposomes as novel delivery systems for lutein. RSC Adv. 2018, 8, 31372–31381. [Google Scholar] [CrossRef]
- Kopec, R.E.; Gleize, B.; Borel, P.; Desmarchelier, C.; Caris-Veyrat, C. Are lutein, lycopene, and beta-carotene lost through the digestive process? Food Funct. 2017, 8, 1494–1503. [Google Scholar] [CrossRef]
- Weigel, F.; Weiss, J.; Decker, E.A.; McClements, D.J. Lutein-enriched emulsion-based delivery systems: Influence of emulsifiers and antioxidants on physical and chemical stability. Food Chem. 2018, 242, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.T.; Lee, T.M.; Chen, B.H. Nonionic microemulsions as solubilizers of hydrophobic drugs: Solubilization of paclitaxel. Materials 2016, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Steiner, B.M.; McClements, D.J.; Davidov-Pardo, G. Encapsulation systems for lutein: A review. Trends Food Sci Technol. 2018, 82, 71–81. [Google Scholar] [CrossRef]
- Vishwanathan, R.; Wilson, T.A.; Nicolosi, R.J. Bioavailability of a nanoemulsion of lutein is greater than a lutein supplement. Nano Biomed. Eng. 2009, 1, 57–73. [Google Scholar] [CrossRef]
- Frede, K.; Henze, A.; Khalil, M.; Baldermann, S.; Schweigert, F.J.; Rawel, H. Stability and cellular uptake of lutein-loaded emulsions. J. Funct. Foods 2014, 8, 118–127. [Google Scholar] [CrossRef]
- Murillo, A.G.; Aguilar, D.; Norris, G.H.; DiMarco, D.M.; Missimer, A.; Hu, S.; Smyth, J.A.; Gannon, S.; Blesso, C.N.; Luo, Y.; et al. Compared with powdered lutein, a lutein nanoemulsion increases plasma and liver lutein, protects against hepatic steatosis, and affects lipoprotein metabolism in guinea pigs. J. Nutr. 2016, 146, 1961–1969. [Google Scholar] [CrossRef]
- Kaur, I.P.; Kakkar, S. Nanotherapy for posterior eye diseases. J. Control. Release 2014, 193, 100–112. [Google Scholar] [CrossRef]
- Reimondez-Troitiño, S.; Csaba, N.; Alonso, M.J.; de la Fuente, M. Nanotherapies for the treatment of ocular diseases. Eur. J. Pharm. Biopharm. 2015, 95, 279–293. [Google Scholar] [CrossRef]
- Lim, C.; Kim, D.-W.; Sim, T.; Hoang, N.H.; Lee, J.W.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Preparation and characterization of a lutein loading nanoemulsion system for ophthalmic eye drops. J. Drug Deliv. Sci. Technol. 2016, 36, 168–174. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, A.; Sun, R.; Xu, J.W.; Yin, T.; He, H.B.; Gou, J.X.; Kong, J.; Zhang, Y.; Tang, X. Penetratin-modified lutein nanoemulsion in-situ gel for the treatment of age-related macular degeneration. Expert Opin. Drug Deliv. 2020, 17, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Patil, D.; Pattewar, S.; Palival, S.; Patil, G.; Sharma, S. Nanostructured lipid carriers: A platform to lipophilic drug for oral bioavailability enhancement. J. Drug Deliv. Ther. 2019, 9, 758–764. [Google Scholar]
- Duong, V.A.; Nguyen, T.T.; Maeng, H.J. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef] [PubMed]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lipid nanocarriers for dermal delivery of lutein: Preparation, characterization, stability and performance. Int. J. Pharm. 2011, 414, 267–275. [Google Scholar] [CrossRef]
- Shah, S.; Bhanderi, B.; Soniwala, M.; Chavda, J. Lutein-loaded solid lipid nanoparticles for ocular delivery: Statistical optimization and ex vivo evaluation. J. Pharm. Innov. 2021, 17, 584–598. [Google Scholar] [CrossRef]
- Tan, F.; Cui, H.; Bai, C.; Qing, C.; Xu, L.; Han, J. Preparation, Optimization, and transcorneal permeability study of Lutein-loaded Solid Lipid Nanoparticles. J. Drug Deliv. Sci. Technol. 2021, 62, 102362. [Google Scholar] [CrossRef]
- Lacatusu, I.; Mitrea, E.; Badea, N.; Stan, R.; Oprea, O.; Meghea, A. Lipid nanoparticles based on omega-3 fatty acids as effective carriers for lutein delivery. Preparation and in vitro characterization studies. J. Funct. Foods 2013, 5, 1260–1269. [Google Scholar] [CrossRef]
- Liu, C.H.; Chiu, H.C.; Wu, W.C.; Sahoo, S.L.; Hsu, C.Y. Novel lutein loaded lipid nanoparticles on porcine corneal distribution. J. Ophthalmol. 2014, 2014, 304694. [Google Scholar] [CrossRef] [Green Version]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Langer, R.; Xinqiao, J. Nanostructured materials for applications in drug delivery and tissue engineering. J. Biomat. Sci. Polym. E 2007, 18, 241–268. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A natural biopolymer with a wide and varied range of applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Pathomthongtaweechai, N.; Muanprasat, C. Potential applications of chitosan-based nanomaterials to surpass the gastrointestinal physiological obstacles and enhance the intestinal drug absorption. Pharmaceutics 2021, 13, 887. [Google Scholar] [CrossRef] [PubMed]
- Mikušová, V.; Mikuš, P. Advances in chitosan-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef]
- Hong, D.Y.; Lee, J.-S.; Lee, H.G. Chitosan/poly-γ-glutamic acid nanoparticles improve the solubility of lutein. Int. J. Biol. Macromol. 2016, 85, 9–15. [Google Scholar] [CrossRef]
- Chaiyasan, W.; Srinivas, S.P.; Tiyaboonchai, W. Crosslinked chitosan-dextran sulfate nanoparticle for improved topical ocular drug delivery. Mol. Vis. 2015, 21, 1224–1234. [Google Scholar]
- Arunkumar, R.; Prashanth, K.V.H.; Baskaran, V. Promising interaction between nanoencapsulated lutein with low molecular weight chitosan: Characterization and bioavailability of lutein in vitro and in vivo. Food Chem. 2013, 141, 327–337. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Mundargi, R.; Babu, V.; Rangaswamy, V.; Patel, P.; Aminabhavi, T. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J. Control. Release 2008, 125, 193–209. [Google Scholar] [CrossRef]
- Kamil, A.; Smith, D.E.; Blumberg, J.B.; Astete, C.; Sabliov, C.; Chen, C.Y.O. Bioavailability and biodistribution of nanodelivered lutein. Food Chem. 2016, 192, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, R.; Prashanth, K.V.; Manabe, Y.; Hirata, T.; Sugawara, T.; Dharmesh, S.M.; Baskaran, V. Biodegradable poly(lactic-co-glycolic acid)-polyethylene glycol nanocapsules: An efficient carrier for improved solubility, bioavailability, and anticancer property of lutein. J. Pharm. Sci. 2015, 104, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Bolla, P.K.; Gote, V.; Singh, M.; Patel, M.; Clark, B.A.; Renukuntla, J. Lutein-loaded, biotin-decorated polymeric nanoparticles enhance lutein uptake in retinal cells. Pharmaceutics 2020, 12, 798. [Google Scholar] [CrossRef] [PubMed]
- Bolla, P.K.; Gote, V.; Singh, M.; Yellepeddi, V.K.; Patel, M.; Pal, D.; Gong, X.; Sambalingam, D.; Renukuntla, J. Preparation and characterization of lutein loaded folate conjugated polymeric nanoparticles. J. Microencapsul. 2020, 37, 502–516. [Google Scholar] [CrossRef]
- Chittasupho, C.; Posritong, P.; Ariyawong, P. Stability, Cytotoxicity, and Retinal Pigment Epithelial Cell Binding of Hyaluronic Acid-Coated PLGA Nanoparticles Encapsulating Lutein. AAPS Pharmscitech 2018, 20, 1–13. [Google Scholar] [CrossRef]
- Dhas, N.; Mehta, T. Cationic biopolymer functionalized nanoparticles encapsulating lutein to attenuate oxidative stress in effective treatment of Alzheimer’s disease: A non-invasive approach. Int. J. Pharm. 2020, 586, 119553. [Google Scholar] [CrossRef]
- Reboul, E. Mechanisms of carotenoid intestinal absorption: Where do we stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, J.J.; Chee, X.Y.J.; Liu, M.H.; Khan, S.A.; Kim, J.E. Encapsulation of lutein via microfluidic technology: Evaluation of stability and in vitro bioaccessibility. Foods 2021, 10, 2646. [Google Scholar] [CrossRef]
- Ranganathan, A.; Hindupur, R.; Vallikannan, B. Biocompatible lutein-polymer-lipid nanocapsules: Acute and subacute toxicity and bioavailability in mice. Mater. Sci. Eng. C 2016, 69, 1318–1327. [Google Scholar] [CrossRef]
- Toragall, V.; Baskaran, V. Chitosan-sodium alginate-fatty acid nanocarrier system: Lutein bioavailability, absorption pharmacokinetics in diabetic rat and protection of retinal cells against H2O2 induced oxidative stress in vitro. Carbohydr. Polym. 2021, 254, 117409. [Google Scholar] [CrossRef]
- Ranganathan, A.; Manabe, Y.; Sugawara, T.; Hirata, T.; Shivanna, N.; & Baskaran, V. Poly(D,L-lactide-co-glycolide)-phospholipid nanocarrier for efficient delivery of macular pigment lutein: Absorption pharmacokinetics in mice and antiproliferative effect in Hep G2 cells. Drug Deliv. Transl. Res. 2019, 9, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Toragall, V.; Jayapala, N.; Vallikannan, B. Chitosan-oleic acid-sodium alginate a hybrid nanocarrier as an efficient delivery system for enhancement of lutein stability and bioavailability. Int. J. Biol. Macromol. 2020, 150, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Shwetha, H.J.; Shilpa, S.; Mukherjee, M.B.; Ambedkar, R.; Raichur, A.M.; Lakshminarayana, R. Fabrication of chitosan nanoparticles with phosphatidylcholine for improved sustain release, basolateral secretion, and transport of lutein in Caco-2 cells. Int. J. Biol. Macromol. 2020, 163, 2224–2235. [Google Scholar] [CrossRef] [PubMed]


| Nanoscale Delivery System | Carrier | Size (nm) | Encapsulation Efficiency (%) | In Vivo Results | Ref. |
|---|---|---|---|---|---|
| Emulsion-based systems | |||||
| NE (medium-chain triglyceride,α-tocopheryl polyethylene glycol succinate) | 254.2 | NA | As compared to lutein powder, the nanoemulsion increased the level of lutein in the liver and plasma by 1.6- and 2-fold, respectively. | [147] | |
| Polymer-based NPs | |||||
| Chitosan (LMWC) | 80–600 | 85 ± 1 | NPs displayed a considerably higher (27.7%) bioavailability than micellar lutein. Moreover, the postprandial lutein level in the liver (53.9%), plasma (54.5%), and eyes (62.8%) of mice fed NPs were found better than that of the control | [168] | |
| PLGA | 124 ± 4 | 52 ± 3 | NPs improve the pharmacokinetics (Cmax and AUC) of lutein in the plasma and promote lutein accumulation in the mesenteric adipose tissue and spleen, compared to free and micellized lutein | [171] | |
| PLGA-PEG | 80–500 (~200) | 88 ± 2 | The postprandial plasma kinetics of an oral dose of lutein from NPs was found to be higher compared with that of micellized lutein | [172] | |
| Polymer/Lipid-Based NPs | |||||
| PLGA-PL | 140 ± 6 | 90 ± 2 | AUC of lutein after the application of a NPs showed 3.91-fold (plasma), 2.89-fold (liver), and 3.12-fold (eyes) higher levels of absorption than micellized lutein | [181] | |
| CHI-OL-ALG | 40–160 (~200) | NA | In the oral pharmacokinetic study, using a single dose via oral gavage, lutein from NPs exhibited a 128.3% improved oral bioavailability compared to micellar lutein | [182] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algan, A.H.; Gungor-Ak, A.; Karatas, A. Nanoscale Delivery Systems of Lutein: An Updated Review from a Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1852. https://doi.org/10.3390/pharmaceutics14091852
Algan AH, Gungor-Ak A, Karatas A. Nanoscale Delivery Systems of Lutein: An Updated Review from a Pharmaceutical Perspective. Pharmaceutics. 2022; 14(9):1852. https://doi.org/10.3390/pharmaceutics14091852
Chicago/Turabian StyleAlgan, Aslihan Hilal, Ayca Gungor-Ak, and Aysegul Karatas. 2022. "Nanoscale Delivery Systems of Lutein: An Updated Review from a Pharmaceutical Perspective" Pharmaceutics 14, no. 9: 1852. https://doi.org/10.3390/pharmaceutics14091852