Non-Invasive Iontophoretic Delivery of Cytochrome c to the Posterior Segment and Determination of Its Ocular Biodistribution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. UHPLC-UV Analytical Method Development
2.3. In Vitro Ocular Biodistribution of Cytochrome c
2.3.1. Ocular Tissue Source
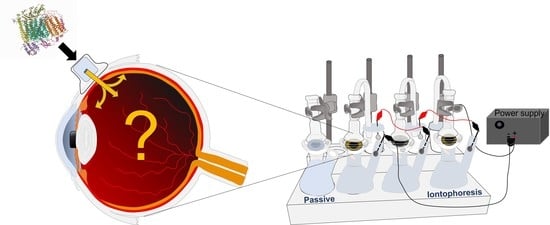
2.3.2. Transscleral Anodal Iontophoresis
Experimental Setup
Effect of Concentration and Iontophoretic Parameters
2.3.3. Post-Iontophoretic Diffusion of Cyt c
2.3.4. Tissue Preparation for Biodistribution Analysis
2.3.5. Determination of the Water Content in Ocular Tissues
2.3.6. Statistical Analysis
3. Results
3.1. Tissue Water Content
3.2. Transscleral Delivery of Cytochrome c
3.2.1. Effect of Concentration
Iontophoresis of Cyt c at Concentrations of 1, 5, and 10 mg/mL (id 3.5 mA/cm2, Duration 4 h)
Iontophoresis of Cyt c at Concentrations of 1, 5 and 10 mg/mL (id 3.5 mA/cm2, Duration 2 h)
Iontophoresis of Cyt c at Concentration of 1 and 5 mg/mL (id 3.5 mA/cm2, Duration 1 h)
3.2.2. Effect of Current Density
Iontophoresis of Cyt c (10 mg/mL) at a Current Density of 5.5 mA/cm2 for 2 h
Iontophoresis of Cyt c (5 mg/mL) at a Current Density of 5.5 mA/cm2 for 2 h
3.2.3. Effect of the Duration of Iontophoresis
Iontophoresis of Cyt c (10 mg/mL) at 3.5 mA/cm2 for 2 h and 4 h
Iontophoresis of Cyt c (5 mg/mL) at 3.5 mA/cm2 for 1 h, 2 h, and 4 h
Iontophoresis of Cyt c (1 mg/mL) at 3.5 mA/cm2 for 1 h, 2 h, and 4 h
3.2.4. Modulation of the Iontophoretic Conditions
3.2.5. Short-Duration Iontophoresis
Iontophoresis of Cyt c (10 mg/mL, id 3.5 mA/cm2) for 10 min
Iontophoresis of Cyt c (10 mg/mL, id 3.5 mA/cm2) for 10 min Followed by Passive Diffusion for 4 h
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jemni-Damer, N.; Guedan-Duran, A.; Fuentes-Andion, M.; Serrano-Bengoechea, N.; Alfageme-Lopez, N.; Armada-Maresca, F.; Guinea, G.V.; Perez-Rigueiro, J.; Rojo, F.; Gonzalez-Nieto, D.; et al. Biotechnology and Biomaterial-Based Therapeutic Strategies for Age-Related Macular Degeneration. Part I: Biomaterials-Based Drug Delivery Devices. Front. Bioeng. Biotechnol. 2020, 8, 549089. [Google Scholar] [CrossRef]
- Amoaku, W.M.; Saker, S.; Stewart, E.A. A review of therapies for diabetic macular oedema and rationale for combination therapy. Eye 2015, 29, 1115–1130. [Google Scholar] [CrossRef]
- Tomita, Y.; Lee, D.K.; Tsubota, K.; Negishi, K.; Kurihara, T. Updates on the Current Treatments for Diabetic Retinopathy and Possibility of Future Oral Therapy. J. Clin. Med. 2021, 10, 4666. [Google Scholar] [CrossRef]
- Kim, Y.C.; Chiang, B.; Wu, X.; Prausnitz, M.R. Ocular delivery of macromolecules. J. Control. Release 2014, 190, 172–181. [Google Scholar] [CrossRef]
- Mandal, A.; Pal, D.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Mitra, A.K. Ocular delivery of proteins and peptides: Challenges and novel formulation approaches. Adv. Drug Deliv. Rev. 2018, 126, 67–95. [Google Scholar] [CrossRef]
- Rodrigues, G.A.; Lutz, D.; Shen, J.; Yuan, X.; Shen, H.; Cunningham, J.; Rivers, H.M. Topical Drug Delivery to the Posterior Segment of the Eye: Addressing the Challenge of Preclinical to Clinical Translation. Pharm. Res. 2018, 35, 245. [Google Scholar] [CrossRef]
- Falavarjani, K.G.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef]
- Gratieri, T.; Santer, V.; Kalia, Y.N. Basic principles and current status of transcorneal and transscleral iontophoresis. Expert Opin. Drug Deliv. 2017, 14, 1091–1102. [Google Scholar] [CrossRef]
- Kalia, Y.N.; Naik, A.; Garrison, J.; Guy, R.H. Iontophoretic drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 619–658. [Google Scholar] [CrossRef]
- Chen, Y.; Kalia, Y.N. Short-duration ocular iontophoresis of ionizable aciclovir prodrugs: A new approach to treat herpes simplex infections in the anterior and posterior segments of the eye. Int. J. Pharm. 2018, 536, 292–300. [Google Scholar] [CrossRef]
- Santer, V.; Del Rio Sancho, S.; Lapteva, M.; Kalia, Y.N. Targeted intracorneal delivery-Biodistribution of triamcinolone acetonide following topical iontophoresis of cationic amino acid ester prodrugs. Int. J. Pharm. 2017, 525, 43–53. [Google Scholar] [CrossRef]
- Cassagne, M.; Laurent, C.; Rodrigues, M.; Galinier, A.; Spoerl, E.; Galiacy, S.D.; Soler, V.; Fournie, P.; Malecaze, F. Iontophoresis Transcorneal Delivery Technique for Transepithelial Corneal Collagen Crosslinking with Riboflavin in a Rabbit Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 594–603. [Google Scholar] [CrossRef]
- Hayden, B.; Jockovich, M.E.; Murray, T.G.; Kralinger, M.T.; Voigt, M.; Hernandez, E.; Feuer, W.; Parel, J.M. Iontophoretic delivery of carboplatin in a murine model of retinoblastoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3717–3721. [Google Scholar] [CrossRef]
- Vaka, S.R.; Sammeta, S.M.; Day, L.B.; Murthy, S.N. Transcorneal iontophoresis for delivery of ciprofloxacin hydrochloride. Curr. Eye Res. 2008, 33, 661–667. [Google Scholar] [CrossRef]
- Cohen, A.E.; Assang, C.; Patane, M.A.; From, S.; Korenfeld, M. Evaluation of dexamethasone phosphate delivered by ocular iontophoresis for treating noninfectious anterior uveitis. Ophthalmology 2012, 119, 66–73. [Google Scholar] [CrossRef]
- Eljarrat-Binstock, E.; Raiskup, F.; Frucht-Pery, J.; Domb, A.J. Transcorneal and transscleral iontophoresis of dexamethasone phosphate using drug loaded hydrogel. J. Control. Release 2005, 106, 386–390. [Google Scholar] [CrossRef]
- Behar-Cohen, F.F.; El Aouni, A.; Gautier, S.; David, G.; Davis, J.; Chapon, P.; Parel, J.M. Transscleral Coulomb-controlled iontophoresis of methylprednisolone into the rabbit eye: Influence of duration of treatment, current intensity and drug concentration on ocular tissue and fluid levels. Exp. Eye Res. 2002, 74, 51–59. [Google Scholar] [CrossRef]
- Eljarrat-Binstock, E.; Orucov, F.; Frucht-Pery, J.; Pe’er, J.; Domb, A.J. Methylprednisolone delivery to the back of the eye using hydrogel iontophoresis. J. Ocul. Pharmacol. Ther. 2008, 24, 344–350. [Google Scholar] [CrossRef]
- Eljarrat-Binstock, E.; Domb, A.J.; Orucov, F.; Frucht-Pery, J.; Pe’er, J. Methotrexate delivery to the eye using transscleral hydrogel iontophoresis. Curr. Eye Res. 2007, 32, 639–646. [Google Scholar] [CrossRef]
- Gungor, S.; Delgado-Charro, M.B.; Ruiz-Perez, B.; Schubert, W.; Isom, P.; Moslemy, P.; Patane, M.A.; Guy, R.H. Trans-scleral iontophoretic delivery of low molecular weight therapeutics. J. Control. Release 2010, 147, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Santer, V.; Chen, Y.; Kalia, Y.N. Controlled non-invasive iontophoretic delivery of triamcinolone acetonide amino acid ester prodrugs into the posterior segment of the eye. Eur. J. Pharm. Biopharm. 2018, 132, 157–167. [Google Scholar] [CrossRef]
- Pescina, S.; Ferrari, G.; Govoni, P.; Macaluso, C.; Padula, C.; Santi, P.; Nicoli, S. In-vitro permeation of bevacizumab through human sclera: Effect of iontophoresis application. J. Pharm. Pharmacol. 2010, 62, 1189–1194. [Google Scholar] [CrossRef]
- Molokhia, S.; Papangkorn, K.; Butler, C.; Higuchi, J.W.; Brar, B.; Ambati, B.; Li, S.K.; Higuchi, W.I. Transscleral Iontophoresis for Noninvasive Ocular Drug Delivery of Macromolecules. J Ocul. Pharmacol. Ther 2020, 36, 247–256. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Tratta, E.; Pescina, S.; Padula, C.; Santi, P.; Nicoli, S. Parameters affecting the transscleral delivery of two positively charged proteins of comparable size. Int. J. Pharm. 2017, 521, 214–221. [Google Scholar] [CrossRef]
- Tratta, E.; Pescina, S.; Padula, C.; Santi, P.; Nicoli, S. In vitro permeability of a model protein across ocular tissues and effect of iontophoresis on the transscleral delivery. Eur. J. Pharm. Biopharm. 2014, 88, 116–122. [Google Scholar] [CrossRef]
- Alvarez-Paggi, D.; Hannibal, L.; Castro, M.A.; Oviedo-Rouco, S.; Demicheli, V.; Tortora, V.; Tomasina, F.; Radi, R.; Murgida, D.H. Multifunctional Cytochrome c: Learning New Tricks from an Old Dog. Chem. Rev. 2017, 117, 13382–13460. [Google Scholar] [CrossRef]
- Cazares-Delgadillo, J.; Naik, A.; Ganem-Rondero, A.; Quintanar-Guerrero, D.; Kalia, Y.N. Transdermal delivery of cytochrome C--A 12.4 kDa protein—across intact skin by constant-current iontophoresis. Pharm. Res. 2007, 24, 1360–1368. [Google Scholar] [CrossRef]
- Heinig, K.; Bertran, E.; Potter, J.; Fraier, D. Ocular bioanalysis: Challenges and advancements in recent years for these rare matrices. Bioanalysis 2017, 9, 1997–2014. [Google Scholar]
- Validation of Analytical Procedures: Text and Methodology Topic Q2 (R1). In International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. 2005. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 1 July 2018).
- Sanchez, I.; Martin, R.; Ussa, F.; Fernandez-Bueno, I. The parameters of the porcine eyeball. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 475–482. [Google Scholar] [CrossRef]
- Crespo-Moral, M.; Garcia-Posadas, L.; Lopez-Garcia, A.; Diebold, Y. Histological and immunohistochemical characterization of the porcine ocular surface. PLoS ONE 2020, 15, e0227732. [Google Scholar] [CrossRef]
- Nicoli, S.; Ferrari, G.; Quarta, M.; Macaluso, C.; Govoni, P.; Dallatana, D.; Santi, P. Porcine sclera as a model of human sclera for in vitro transport experiments: Histology, SEM, and comparative permeability. Mol. Vis. 2009, 15, 259–266. [Google Scholar]
- Pescina, S.; Padula, C.; Santi, P.; Nicoli, S. Effect of formulation factors on the trans-scleral iontophoretic and post-iontophoretic transports of a 40 kDa dextran in vitro. Eur. J. Pharm. Sci. 2011, 42, 503–508. [Google Scholar] [CrossRef]
- Kao, J.C.; Geroski, D.H.; Edelhauser, H.F. Transscleral permeability of fluorescent-labeled antibiotics. J. Ocul. Pharmacol. Ther. 2005, 21, 1–10. [Google Scholar] [CrossRef]
- Taylor, Z.D.; Garritano, J.; Sung, S.; Bajwa, N.; Bennett, D.B.; Nowroozi, B.; Tewari, P.; Sayre, J.; Hubschman, J.P.; Deng, S.; et al. THz and mm-Wave Sensing of Corneal Tissue Water Content: Electromagnetic Modeling and Analysis. IEEE Trans. Terahertz Sci. Technol. 2015, 5, 170–183. [Google Scholar] [CrossRef]
- Edwards, A.; Prausnitz, M.R. Fiber matrix model of sclera and corneal stroma for drug delivery to the eye. AIChE J. 1998, 44, 214–225. [Google Scholar] [CrossRef]
- Le Goff, M.M.; Bishop, P.N. Adult vitreous structure and postnatal changes. Eye 2008, 22, 1214–1222. [Google Scholar] [CrossRef]
- Kim, S.H.; Lutz, R.J.; Wang, N.S.; Robinson, M.R. Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res. 2007, 39, 244–254. [Google Scholar] [CrossRef]
- Dubey, S.; Kalia, Y.N. Understanding the poor iontophoretic transport of lysozyme across the skin: When high charge and high electrophoretic mobility are not enough. J. Control. Release 2014, 183, 35–42. [Google Scholar] [CrossRef]
- Srikantha, N.; Mourad, F.; Suhling, K.; Elsaid, N.; Levitt, J.; Chung, P.H.; Somavarapu, S.; Jackson, T.L. Influence of molecular shape, conformability, net surface charge, and tissue interaction on transscleral macromolecular diffusion. Exp. Eye Res. 2012, 102, 85–92. [Google Scholar] [CrossRef]
- Hämäläinen, K.M.; Kananen, K.; Auriola, S.; Kontturi, K.; Urtti, A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Investig. Ophthalmol. Vis. Sci. 1997, 38, 627–634. [Google Scholar]
- Kevin Li, S.; Hao, J.; Liddell, M. Chapter 11—Electrotransport Across Membranes in Biological Media: Electrokinetic Theories and Applications in Drug Delivery. In Transport in Biological Media; Becker, S.M., Kuznetsov, A.V., Eds.; Elsevier: Boston, MA, USA, 2013; pp. 417–454. [Google Scholar]
- Chopra, P.; Hao, J.; Li, S.K. Iontophoretic transport of charged macromolecules across human sclera. Int. J. Pharm. 2010, 388, 107–113. [Google Scholar] [CrossRef]
- Wen, H.; Hao, J.; Li, S.K. Characterization of human sclera barrier properties for transscleral delivery of bevacizumab and ranibizumab. J. Pharm. Sci. 2013, 102, 892–903. [Google Scholar] [CrossRef]
- Ambati, J.; Canakis, C.S.; Miller, J.W.; Gragoudas, E.S.; Edwards, A.; Weissgold, D.J.; Kim, I.; Delori, F.C.; Adamis, A.P. Diffusion of high molecular weight compounds through sclera. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1181–1185. [Google Scholar]
- Pescina, S.; Govoni, P.; Antopolsky, M.; Murtomaki, L.; Padula, C.; Santi, P.; Nicoli, S. Permeation of proteins, oligonucleotide and dextrans across ocular tissues: Experimental studies and a literature update. J. Pharm. Sci. 2015, 104, 2190–2202. [Google Scholar] [CrossRef]
- Amrite, A.; Pugazhenthi, V.; Cheruvu, N.; Kompella, U. Delivery of celecoxib for treating diseases of the eye: Influence of pigment and diabetes. Expert Opin. Drug Deliv. 2010, 7, 631–645. [Google Scholar] [CrossRef]
- Abrahamsson, T.; Bostrom, S.; Brautigam, J.; Lagerstrom, P.O.; Regardh, C.G.; Vauqelin, G. Binding of the beta-blockers timolol and H 216/44 to ocular melanin. Exp. Eye Res. 1988, 47, 565–577. [Google Scholar] [CrossRef]
- Molokhia, S.A.; Jeong, E.K.; Higuchi, W.I.; Li, S.K. Transscleral iontophoretic and intravitreal delivery of a macromolecule: Study of ocular distribution in vivo and postmortem with MRI. Exp. Eye Res. 2009, 88, 418–425. [Google Scholar] [CrossRef] [Green Version]







| [Cyt c] (mg/mL) | Duration b (h) | Current Density (mA/cm2) |
|---|---|---|
| 1 | 1, 2, 4 | 3.5 |
| 5 | ||
| 10 | 2, 4 | |
| 5 | 2 | 5.5 |
| 10 | ||
| 10 c | 10 min and | 3.5 |
| 10 min + 4 h passive diffusion |
| Tissue | Experimentally Determined Water Content (%) | Reported Water Content (%) |
|---|---|---|
| Cornea (CO) | 71.3 ± 6.9 | 72–78 [30,35] |
| Aqueous humor (AH) | 99 ± 0.3 | NR |
| Iris and Ciliary body (IR) | 58.8 ± 7.2 | NR |
| Retina (RE) | 92.4 ± 0.5 | NR |
| Choroid (CH) | 70 ± 2.5 | NR |
| Sclera (SC) | 61.4 ± 6.2 | 65–75 [30,36] |
| Vitreous humor (VH) | 99.2 ± 0.3 | 98–99.7 [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González Iglesias, L.G.; Messaoudi, S.; Kalia, Y.N. Non-Invasive Iontophoretic Delivery of Cytochrome c to the Posterior Segment and Determination of Its Ocular Biodistribution. Pharmaceutics 2022, 14, 1832. https://doi.org/10.3390/pharmaceutics14091832
González Iglesias LG, Messaoudi S, Kalia YN. Non-Invasive Iontophoretic Delivery of Cytochrome c to the Posterior Segment and Determination of Its Ocular Biodistribution. Pharmaceutics. 2022; 14(9):1832. https://doi.org/10.3390/pharmaceutics14091832
Chicago/Turabian StyleGonzález Iglesias, Laura Gisela, Siwar Messaoudi, and Yogeshvar N. Kalia. 2022. "Non-Invasive Iontophoretic Delivery of Cytochrome c to the Posterior Segment and Determination of Its Ocular Biodistribution" Pharmaceutics 14, no. 9: 1832. https://doi.org/10.3390/pharmaceutics14091832