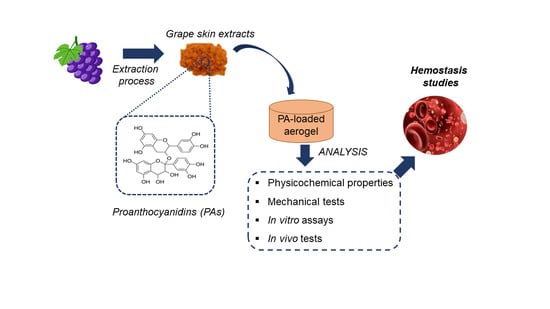
The Effect on Hemostasis of Gelatin-Graphene Oxide Aerogels Loaded with Grape Skin Proanthocyanidins: In Vitro and In Vivo Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PA Extraction from País Grape Skin
2.3. Purification of PAs
2.4. Characterization of PAs
2.5. Loading PA into Aerogels
2.6. Influence of PAs on Aerogel Properties
2.7. In Vitro Hemostatic Studies
2.8. In Vivo Hemostatic Assays
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of PAs
3.2. Influence of PA Loading on the Aerogel Properties
3.3. In Vitro Hemostatic Potential of PAs
3.4. In Vivo Hemostatic Potential of PAs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; You, X.; Dai, C.; Tong, T.; Wu, J. Hemostatic Nanotechnologies for External and Internal Hemorrhage Management. Biomater. Sci. 2020, 8, 4396–4412. [Google Scholar] [CrossRef]
- Sepúlveda, C.; Palomo, I.; Fuentes, E. Primary and Secondary Haemostasis Changes Related to Aging. Mech. Ageing Dev. 2015, 150, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Bobrowski, M.; Borowiecka, M.; Podsędek, A.; Golański, J.; Nowak, P. Anticoagulant Effect of Polyphenols-Rich Extracts from Black Chokeberry and Grape Seeds. Fitoterapia 2011, 82, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Sinegre, T.; Teissandier, D.; Milenkovic, D.; Morand, C.; Lebreton, A. Epicatechin Influences Primary Hemostasis, Coagulation and Fibrinolysis. Food Funct. 2019, 10, 7291–7298. [Google Scholar] [CrossRef] [PubMed]
- Mangels, D.R.; Mohler, E.R. Catechins as Potential Mediators of Cardiovascular Health. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 757–763. [Google Scholar] [CrossRef]
- Mellado, C.; Figueroa, T.; Baez, R.; Castillo, R.; Melendrez, M.; Schulz, B.; Fernandez, K. Development of Graphene Oxide Composite Aerogel with Proanthocyanidins with Hemostatic Properties As a Delivery System. ACS Appl. Mater. Interfaces 2018, 10, 7717–7729. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in Grape Seeds: An Updated Review of Their Health Benefits and Potential Uses in the Food Industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, H. Alginate/Pectin Aerogel Microspheres for Controlled Release of Proanthocyanidins. Int. J. Biol. Macromol. 2019, 136, 936–943. [Google Scholar] [CrossRef]
- Fernández, K.; Aburto, J.; von Plessing, C.; Rockel, M.; Aspé, E. Factorial Design Optimization and Characterization of Poly-Lactic Acid (PLA) Nanoparticle Formation for the Delivery of Grape Extracts. Food Chem. 2016, 207, 75–85. [Google Scholar] [CrossRef]
- Eriz, G.; Sanhueza, V.; Roeckel, M.; Fernández, K. Inhibition of the Angiotensin-Converting Enzyme by Grape Seed and Skin Proanthocyanidins Extracted from Vitis vinífera L. cv. País. LWT Food Sci. Technol. 2011, 44, 860–865. [Google Scholar] [CrossRef]
- Borges-Vilches, J.; Figueroa, T.; Guajardo, S.; Aguayo, C.; Fernández, K. Improved Hemocompatibility for Gelatin-Graphene Oxide Composite Aerogels Reinforced with Proanthocyanidins for Wound Dressing Applications. Colloids Surf. B Biointerfaces 2021, 206, 111941. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, T.; Carmona, S.; Guajardo, S.; Borges, J.; Aguayo, C.; Fernández, K. Synthesis and Characterization of Graphene Oxide Chitosan Aerogels Reinforced with Flavan-3-Ols as Hemostatic Agents. Colloids Surf. B Biointerfaces 2021, 197, 111398. [Google Scholar] [CrossRef]
- Chira, K.; Schmauch, G.; Saucier, C.; Fabre, S.; Teissedre, P.-L. Grape Variety Effect on Proanthocyanidin Composition and Sensory Perception of Skin and Seed Tannin Extracts from Bordeaux Wine Grapes (Cabernet Sauvignon and Merlot) for Two Consecutive Vintages (2006 and 2007). J. Agric. Food Chem. 2009, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Locilento, D.A.; Mercante, L.A.; Andre, R.S.; Mattoso, L.H.C.; Luna, G.L.F.; Brassolatti, P.; Anibal, F.D.F.; Correa, D.S. Biocompatible and Biodegradable Electrospun Nanofibrous Membranes Loaded with Grape Seed Extract for Wound Dressing Application. J. Nanomater. 2019, 2019, 2472964. Available online: https://www.hindawi.com/journals/jnm/2019/2472964/ (accessed on 31 July 2020). [CrossRef]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef] [PubMed]
- Rinki, K.; Dutta, P.K.; Hunt, A.J.; Macquarrie, D.J.; Clark, J.H. Chitosan Aerogels Exhibiting High Surface Area for Biomedical Application: Preparation, Characterization, and Antibacterial Study. Int. J. Polym. Mater. 2011, 60, 988–999. [Google Scholar] [CrossRef]
- Bijak, M.; Kolodziejczyk-Czepas, J.; Ponczek, M.B.; Saluk, J.; Nowak, P. Protective Effects of Grape Seed Extract against Oxidative and Nitrative Damage of Plasma Proteins. Int. J. Biol. Macromol. 2012, 51, 183–187. [Google Scholar] [CrossRef]
- Xu, L.Q.; Neoh, K.-G.; Kang, E.-T. Natural Polyphenols as Versatile Platforms for Material Engineering and Surface Functionalization. Prog. Polym. Sci. 2018, 87, 165–196. [Google Scholar] [CrossRef]
- Unalan, I.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Frank, G.; Boccaccini, A.R. Physical and Antibacterial Properties of Peppermint Essential Oil Loaded Poly (ε-Caprolactone) (PCL) Electrospun Fiber Mats for Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, T.; Aguayo, C.; Fernández, K. Design and Characterization of Chitosan-Graphene Oxide Nanocomposites for the Delivery of Proanthocyanidins. Int. J. Nanomed. 2020, 15, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Borges-Vilches, J. Development and Validation of a New Hemostatic Device Based on a Gelatin–Graphene Oxide Aerogel Rein Forced with Grape Skin Extracts for Use as a Wound Dressing Material. Ph.D. Thesis, Universidad de Concepción, Concepción, Chile, 2022. Available online: http://repositorio.udec.cl/jspui/bitstream/11594/9869/1/Tesis%20Jessica%20Borges.pdf (accessed on 31 July 2020).
- Morales, C.; Roeckel, M.; Fernández, K. Microscopic Modeling of País Grape Seed Extract Absorption in the Small Intestine. AAPS PharmSciTech 2014, 15, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Jerez, M.; Selga, A.; Sineiro, J.; Torres, J.L.; Núñez, M.J. A Comparison between Bark Extracts from Pinus Pinaster and Pinus Radiata: Antioxidant Activity and Procyanidin Composition. Food Chem. 2007, 100, 439–444. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Analysis of Proanthocyanidin Cleavage Products Following Acid-Catalysis in the Presence of Excess Phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.M.; Porter, L.J.; Hemingway, R.W. Molecular Weight Profiles of Proanthocyanidin Polymers. Phytochemistry 1983, 22, 569–572. [Google Scholar] [CrossRef]
- Borges-Vilches, J.; Figueroa, T.; Guajardo, S.; Meléndrez, M.; Fernández, K. Development of Gelatin Aerogels Reinforced with Graphene Oxide by Microwave-Assisted Synthesis: Influence of the Synthesis Conditions on Their Physicochemical Properties. Polymer 2020, 208, 122951. [Google Scholar] [CrossRef]
- Kang, P.-L.; Chang, S.J.; Manousakas, I.; Lee, C.W.; Yao, C.-H.; Lin, F.-H.; Kuo, S.M. Development and Assessment of Hemostasis Chitosan Dressings. Carbohydr. Polym. 2011, 85, 565–570. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Niu, X.; Zhou, G.; Li, P.; Fan, Y. Improved Hemocompatibility and Endothelialization of Vascular Grafts by Covalent Immobilization of Sulfated Silk Fibroin on Poly(Lactic-Co-Glycolic Acid) Scaffolds. Biomacromolecules 2011, 12, 2914–2924. [Google Scholar] [CrossRef]
- Feng, C.; Li, J.; Wu, G.S.; Mu, Y.Z.; Kong, M.; Jiang, C.Q.; Cheng, X.J.; Liu, Y.; Chen, X.G. Chitosan-Coated Diatom Silica as Hemostatic Agent for Hemorrhage Control. ACS Appl. Mater. Interfaces 2016, 8, 34234–34243. [Google Scholar] [CrossRef]
- Chen, J.; Lv, L.; Li, Y.; Ren, X.; Luo, H.; Gao, Y.; Yan, H.; Li, Y.; Qu, Y.; Yang, L.; et al. Preparation and Evaluation of Bletilla striata Polysaccharide/Graphene Oxide Composite Hemostatic Sponge. Int. J. Biol. Macromol. 2019, 130, 827–835. [Google Scholar] [CrossRef]
- Li, G.; Quan, K.; Xu, C.; Deng, B.; Wang, X. Synergy in Thrombin-Graphene Sponge for Improved Hemostatic Efficacy and Facile Utilization. Colloids Surf. B Biointerfaces 2018, 161, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Quan, K.; Li, G.; Luan, D.; Yuan, Q.; Tao, L.; Wang, X. Black Hemostatic Sponge Based on Facile Prepared Cross-Linked Graphene. Colloids Surf. B Biointerfaces 2015, 132, 27–33. [Google Scholar] [CrossRef]
- Quan, K.; Li, G.; Tao, L.; Xie, Q.; Yuan, Q.; Wang, X. Diaminopropionic Acid Reinforced Graphene Sponge and Its Use for Hemostasis. ACS Appl. Mater. Interfaces 2016, 8, 7666–7673. [Google Scholar] [CrossRef] [PubMed]
- Fernández, K.; Agosin, E. Quantitative Analysis of Red Wine Tannins Using Fourier-Transform Mid-Infrared Spectrometry. J. Agric. Food Chem. 2007, 55, 7294–7300. [Google Scholar] [CrossRef] [PubMed]
- Ringwald, C.; Ball, V. Step-by-Step Deposition of Type B Gelatin and Tannic Acid Displays a Peculiar Ionic Strength Dependence at PH 5. RSC Adv. 2016, 6, 4730–4738. [Google Scholar] [CrossRef]
- Cha, C.; Shin, S.R.; Gao, X.; Annabi, N.; Dokmeci, M.R.; Tang, X.S.; Khademhosseini, A. Controlling Mechanical Properties of Cell-Laden Hydrogels by Covalent Incorporation of Graphene Oxide. Small 2014, 10, 514–523. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.; Shin, Y.C.; Kim, M.J.; Park, J.H.; Hong, S.W.; Kim, B.; Oh, J.-W.; Park, K.D.; Han, D.-W. In Situ Forming Gelatin/Graphene Oxide Hydrogels for Facilitated C2C12 Myoblast Differentiation. Appl. Spectrosc. Rev. 2016, 51, 527–539. [Google Scholar] [CrossRef]
- Mohamed, E.; Fitzgerald, A.; Tsuzuki, T. The Role of Nanoscale Structures in the Development of Topical Hemostatic Agents. Mater. Today Nano 2021, 16, 100137. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, S.; Zhu, X.; Zhao, Y.; Chen, Y.; Tong, J.; Shi, X.; Du, Y.; Zhong, Z.; Ye, Q. Emerging Chitin Nanogels/Rectorite Nanocomposites for Safe and Effective Hemorrhage Control. J. Mater. Chem. B 2019, 7, 5096–5103. [Google Scholar] [CrossRef]
- Chen, Z.; Han, L.; Liu, C.; Du, Y.; Hu, X.; Du, G.; Shan, C.; Yang, K.; Wang, C.; Li, M.; et al. A Rapid Hemostatic Sponge Based on Large, Mesoporous Silica Nanoparticles and N-Alkylated Chitosan. Nanoscale 2018, 10, 20234–20245. [Google Scholar] [CrossRef]
- Sperling, C.; Fischer, M.; Maitz, M.F.; Werner, C. Blood Coagulation on Biomaterials Requires the Combination of Distinct Activation Processes. Biomaterials 2009, 30, 4447–4456. [Google Scholar] [CrossRef]
- Mani, M.P.; Jaganathan, S.K. Fabrication and Characterization of Electrospun Polyurethane Blended with Dietary Grapes for Skin Tissue Engineering. J. Ind. Text. 2020, 50, 655–674. [Google Scholar] [CrossRef]
- Li, G.; Liang, Y.; Xu, C.; Sun, H.; Tao, L.; Wei, Y.; Wang, X. Polydopamine Reinforced Hemostasis of a Graphene Oxide Sponge via Enhanced Platelet Stimulation. Colloids Surf. B Biointerfaces 2019, 174, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic Biochemical Mechanisms behind the Health Benefits of Polyphenols. Mol. Aspects Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O.; Dufour, C.; Manach, C.; Morand, C.; Remesy, C. Binding of Flavonoids to Plasma Proteins. Methods Enzymol. 2001, 335, 319–333. [Google Scholar]
- Brash, J.L.; Horbett, T.A.; Latour, R.A.; Tengvall, P. The Blood Compatibility Challenge. Part 2: Protein Adsorption Phenomena Governing Blood Reactivity. Acta Biomater. 2019, 94, 11–24. [Google Scholar] [CrossRef]
- Gorbet, M.; Sperling, C.; Maitz, M.F.; Siedlecki, C.A.; Werner, C.; Sefton, M.V. The Blood Compatibility Challenge. Part 3: Material Associated Activation of Blood Cascades and Cells. Acta Biomater. 2019, 94, 25–32. [Google Scholar] [CrossRef]
- Mann, K.G.; Brummel, K.; Butenas, S. What Is All That Thrombin For? J. Thromb. Haemost. 2003, 1, 1504–1514. [Google Scholar] [CrossRef]
- Žitek, T.; Dariš, B.; Finšgar, M.; Knez, Ž.; Bjelić, D.; Knez Hrnčič, M. The Effect of Polyphenolics in Extracts from Natural Materials on Metabolic Activity of Metastatic Melanoma WM-266-4 Cells. Appl. Sci. 2020, 10, 3499. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and Its Bioactive Constituents: An Update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape Seed Extract: Having a Potential Health Benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Ulusoy, S.; Orem, A.; Ersoz, S.; Alkanat, M.; Yucesan, F.B.; Kaynar, K.; Al, S. Protective Effect of the Grape Seed Proanthocyanidin Extract in a Rat Model of Contrast-Induced Nephropathy. Kidney Blood Press. Res. 2012, 35, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, Y.; Liu, H.; Hu, N.; Zhang, Y.; Wang, S. Grape Seed Extract Ameliorates PhIP-Induced Colonic Injury by Modulating Gut Microbiota, Lipid Metabolism, and NF-ΚB Signaling Pathway in Rats. J. Funct. Foods 2021, 78, 104362. [Google Scholar] [CrossRef]
- Ben Youssef, S.; Brisson, G.; Doucet-Beaupré, H.; Castonguay, A.-M.; Gora, C.; Amri, M.; Lévesque, M. Neuroprotective Benefits of Grape Seed and Skin Extract in a Mouse Model of Parkinson’s Disease. Nutr. Neurosci. 2021, 24, 197–211. [Google Scholar] [CrossRef]
- Eid, R.A.; Zaki, M.S.A.; Al-Shraim, M.; Eldeen, M.A.; Haidara, M.A. Grape Seed Extract Protects against Amiodarone-Induced Nephrotoxicity and Ultrastructural Alterations Associated with the Inhibition of Biomarkers of Inflammation and Oxidative Stress in Rats. Ultrastruct. Pathol. 2021, 45, 49–58. [Google Scholar] [CrossRef]
- Soltani, R.; Haghighat, A.; Fanaei, M.; Asghari, G. Evaluation of the Effect of Green Tea Extract on the Prevention of Gingival Bleeding after Posterior Mandibular Teeth Extraction: A Randomized Controlled Trial. Evid. Based Complementary Altern. Med. 2014, 2014, e857651. [Google Scholar] [CrossRef]
- Kalalinia, F.; Amiri, N.; Mehrvarzian, N.; Fazly Bazzaz, B.S.; Iranshahi, M.; Shahroodi, A.; Arabzadeh, S.; Abbaspour, M.; Badiee Aaval, S.; Movaffagh, J. Topical Green Tea Formulation with Anti-Hemorrhagic and Antibacterial Effects. Iran. J. Basic Med. Sci. 2020, 23, 1085–1090. [Google Scholar] [CrossRef]




| Compounds | Content (µM) |
|---|---|
| (+)-catechin (C) | 156 ± 22 |
| (−)-epicatechin (EC) | not detected |
| epigallocatechin (ECG) | not detected |
| (+)-catechin-phloroglucinol (C-P) | 92 ± 5 |
| (−)-epicatechin-phloroglucinol (EC-P) | 2900 ± 152 |
| epicatechin gallate-phloroglucinol (ECG-P) | 267 ± 15 |
| epigallocatechin-phloroglucinol (EGC-P) | 574 ± 46 |
| Molecules Percentage (%) | Molecular Weight (g/mol) |
|---|---|
| 10.5 | 25,807–14,149 |
| 22.7 | 14,149–8866 |
| 55.9 | 8866–1582 |
| 3.8 | 1582–1156 |
| 3.9 | 1156–791 |
| 3.1 | 791–451 |
| Aerogels | Apparent Porosity (%) | Elastic Modulus (kPa) | Surface Charge (mV) | Blood Coagulation (%) |
|---|---|---|---|---|
| Unloaded aerogel | 91.8 ± 1.4 a | 2.5 ± 0.6 a | −3.9 ± 1.1 a | 18 ± 2.6 a |
| PA-loaded aerogel | 91.3 ± 1.2 a | 2.8 ± 0.3 a | −5.9 ± 1.2 a | 48 ± 3.4 b |
| Samples | aPTT (s) | PT (s) | P-Selectin Levels (ng/mL) |
|---|---|---|---|
| Unloaded aerogel | 33.3 ± 0.8 a | 13.2 ± 0.8 a | 124.6 ± 3.2 a |
| PA-loaded aerogel | 33.9 ± 0.4 a | 13.1 ± 0.2 a | 92.4 ± 1.9 b |
| PA from grape skin | 40.2 ± 0.8 b | 13.1 ± 0.6 a | - |
| Control plasma | 31 ± 0.5 c | 11.9 ± 0.5 b | 216.5 ± 2.0 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges-Vilches, J.; Aguayo, C.; Fernández, K. The Effect on Hemostasis of Gelatin-Graphene Oxide Aerogels Loaded with Grape Skin Proanthocyanidins: In Vitro and In Vivo Evaluation. Pharmaceutics 2022, 14, 1772. https://doi.org/10.3390/pharmaceutics14091772
Borges-Vilches J, Aguayo C, Fernández K. The Effect on Hemostasis of Gelatin-Graphene Oxide Aerogels Loaded with Grape Skin Proanthocyanidins: In Vitro and In Vivo Evaluation. Pharmaceutics. 2022; 14(9):1772. https://doi.org/10.3390/pharmaceutics14091772
Chicago/Turabian StyleBorges-Vilches, Jessica, Claudio Aguayo, and Katherina Fernández. 2022. "The Effect on Hemostasis of Gelatin-Graphene Oxide Aerogels Loaded with Grape Skin Proanthocyanidins: In Vitro and In Vivo Evaluation" Pharmaceutics 14, no. 9: 1772. https://doi.org/10.3390/pharmaceutics14091772