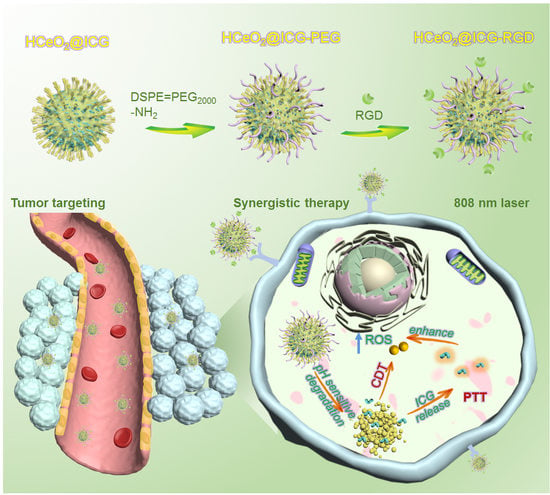
Hollow Mesoporous CeO2-Based Nanoenzymes Fabrication for Effective Synergistic Eradication of Malignant Breast Cancer via Photothermal–Chemodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus-like Mesoporous Silica Synthesis
2.2. HCeO2@ICG Synthesis
2.3. HCeO2@ICG-RGD Synthesis
2.4. Cell Targeting and Intracellular ROS Generation Studies
2.5. Fluorescent Imaging of Tumor Targeting and Photothermal Imaging In Vivo
2.6. Synergistic Antitumor Studies of HCeO2@ICG-RGD
3. Results and Discussion
3.1. HCeO2@ICG-RGD Fabrication
3.2. HCeO2@ICG-RGD Characterization
3.3. Cellular Uptake, ROS Generation, and Cell Killing Effect
3.4. Tumor Targeting and Tumor Inhibition Capability In Vivo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Tuy, K.; Rickenbacker, L.; Hjelmeland, A.B. Reactive oxygen species produced by altered tumor metabolism impacts cancer stem cell maintenance. Redox Biol. 2021, 44, 101953. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting Cancer cells by ROS-mediated mechanisms: A radical therapeutic approach. Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, F.; Tan, Y.; Wen, L.; Li, Y.; Yuan, H.; Hu, F. Guiding appropriate timing of laser irradiation by polymeric micelles for maximizing chemo-photodynamic therapy. Int. J. Nanomed. 2020, 15, 6531–6543. [Google Scholar] [CrossRef]
- An, J.; Hu, Y.G.; Cheng, K.; Li, C.; Hou, X.L.; Wang, G.L.; Zhang, X.S.; Liu, B.; Zhao, Y.D.; Zhang, M.Z. ROS-augmented and tumor-microenvironment responsive biodegradable nanoplatform for enhancing chemo-sonodynamic therapy. Biomaterials 2020, 234, 119761. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of nanomaterials in biomedical imaging and cancer therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Tang, X.L.; Wang, Z.; Zhu, Y.Y.; Xiao, H.; Xiao, Y.; Cui, S.; Lin, B.L.; Yang, K.; Liu, H.Y. Hypoxia-activated ROS burst liposomes boosted by local mild hyperthermia for photo/chemodynamic therapy. J. Control. Release 2020, 328, 100–111. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.X.; Wan, S.S.; Zhang, X.Z. Nanocatalyst-mediated chemodynamic tumor therapy. Adv. Healthc. Mater. 2022, 11, e2101971. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic therapy: Tumour microenvironment-mediated Fenton and Fenton-like reactions. Angew. Chem. Int. Ed. 2019, 58, 946–956. [Google Scholar] [CrossRef]
- Yu, Z.; Lou, R.; Pan, W.; Li, N.; Tang, B. Nanoenzymes in disease diagnosis and therapy. Chem. Commun. 2020, 56, 15513–15524. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, Y.; Shi, J. Nanoenzyme-augmented cancer sonodynamic therapy by catalytic tumor oxygenation. ACS Nano 2018, 12, 3780–3795. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Guo, X.; Zhou, H.; Ren, J.; Li, Y.; Duan, S.; Gong, X.; Du, B. Mild Acid-responsive “nanoenzyme capsule” remodeling of the tumor microenvironment to increase tumor penetration. ACS Appl. Mater. Interfaces 2020, 12, 20214–20227. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wu, X.; Cheng, Y.; Yan, J.; Feng, Y.; Chen, R.; Zheng, R.; Li, X.; Song, P.; Wang, Y.; et al. A biomimetic nanoenzyme for starvation therapy enhanced photothermal and chemodynamic tumor therapy. Nanoscale 2020, 12, 23159–23165. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Wang, L.; Xie, Z. Endogenous hydrogen sulfide-triggered MOF-based nanoenzyme for synergic cancer therapy. ACS Appl. Mater. Interfaces 2020, 12, 30213–30220. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, J.; Zhang, L.; Zhang, C.; Qiu, Z.; Zhao, S.; Huang, Y.; Liang, H. A unique multifunctional nanoenzyme tailored for triggering tumor microenvironment activated NIR-II photoacoustic imaging and chemodynamic/photothermal combined therapy. Adv. Healthc. Mater. 2022, 11, e2102073. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, H.; Lim, W.Q.; Phua, S.; Xu, P.; Chen, Q.; Guo, Z.; Zhao, Y. A Mesoporous nanoenzyme derived from metal-organic frameworks with endogenous oxygen generation to alleviate tumor hypoxia for significantly enhanced photodynamic therapy. Adv. Mater. 2019, 31, e1901893. [Google Scholar] [CrossRef]
- Li, J.; Xie, C.; Huang, J.; Jiang, Y.; Miao, Q.; Pu, K. Semiconducting Polymer Nanoenzymes with Photothermic Activity for Enhanced Cancer Therapy. Angew. Chem. Int. Ed. 2018, 57, 3995–3998. [Google Scholar] [CrossRef]
- Zeng, L.; Cheng, H.; Dai, Y.; Su, Z.; Wang, C.; Lei, L.; Lin, D.; Li, X.; Chen, H.; Fan, K.; et al. In vivo regenerable cerium oxide nanozyme-loaded pH/H2O2-responsive nanovesicle for tumor-targeted photothermal and photodynamic therapies. ACS Appl. Mater. Interfaces 2021, 13, 233–244. [Google Scholar] [CrossRef]
- Fan, Y.; Li, P.; Hu, B.; Liu, T.; Tang, Y. A Smart photosensitizer-cerium oxide nanoprobe for highly selective and efficient photodynamic therapy. Inorg. Chem. 2019, 58, 7295–7302. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.; Chen, F. Improving the catalytic activity of CeO2/H2O2 system by sulfation pretreatment of CeO2. J. Mol. Catal. A Chem. 2013, 381, 38–45. [Google Scholar] [CrossRef]
- Fu, L.H.; Wan, Y.; Qi, C.; He, J.; Li, C.; Yang, C.; Xu, H.; Lin, J.; Huang, P. Nanocatalytic theranostics with glutathione depletion and enhanced reactive oxygen species generation for efficient cancer therapy. Adv. Mater. 2021, 33, e2006892. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, H.; Sun, B.; Du, S.; Cui, S.; Zhang, L.; Ding, N.; Yang, D. pH-responsive oxygen and hydrogen peroxide self-supplying nanosystem for photodynamic and chemodynamic therapy of wound infection. ACS Appl. Mater. Interfaces 2021, 13, 59720–59730. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, T.; Han, F.; Wang, Y.; Gan, Y.; Shi, J.; Wang, T.; Akhtar, M.L.; Li, Y. A cascade-reaction enabled synergistic cancer starvation/ROS-mediated/chemo-therapy with an enzyme modified Fe-based MOF. Biomater. Sci. 2019, 7, 3683–3692. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yin, C.; Zhang, Y.; Liu, Y.; Huang, W. “Dual lock-and-key”-controlled ceria nanotubes-based nanozymes for tumor-specific photothermal therapy. Dyes Pigment. 2021, 191, 109350. [Google Scholar] [CrossRef]
- Zheng, X.; Xing, D.; Zhou, F.; Wu, B.; Chen, W.R. Indocyanine green-containing nanostructure as near infrared dual-functional targeting probes for optical imaging and photothermal therapy. Mol. Pharm. 2011, 8, 447–456. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, L.; Zhu, Y.; Wang, S.; Shen, G.; Jin, L.; Liang, R. Chemodynamic/photothermal synergistic therapy based on Ce-doped Cu-Al layered double hydroxides. J. Mater. Chem. B 2021, 9, 710–718. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, H.; Lim, J.; Park, J. Liposomal indocyanine green for enhanced photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 5683–5691. [Google Scholar] [CrossRef]
- Li, X.; Wei, Z.; Bie, N.; Zhang, X.; Zhan, G.; Li, J.; Qin, J.; Yu, J.; Zhang, B.; Gan, L.; et al. Reversing insufficient photothermal therapy-induced tumor relapse and metastasis by regulating cancer-associated fibroblasts. Nat. Commun. 2022, 13, 2794. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, Y.; Du, W.; Liu, Z.; Zhang, S.; Yang, J.; Yao, H.; Liu, T.; Ma, M.; Chen, H. Clearable theranostic platform with a pH-independent chemodynamic therapy enhancement strategy for synergetic photothermal tumor therapy. ACS Appl. Mater. Interfaces 2019, 11, 18133–18144. [Google Scholar] [CrossRef]
- Huo, M.; Wang, L.; Wang, Y.; Chen, Y.; Shi, J. Nanocatalytic tumor therapy by single-atom catalysts. ACS Nano 2019, 13, 2643–2653. [Google Scholar] [CrossRef]
- Nie, X.; Xia, L.; Wang, H.L.; Chen, G.; Wu, B.; Zeng, T.Y.; Hong, C.Y.; Wang, L.H.; You, Y.Z. Photothermal therapy nanomaterials boosting transformation of Fe(III) into Fe(II) in tumor cells for highly improving chemodynamic therapy. ACS Appl. Mater. Interfaces 2019, 11, 31735–31742. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Luo, G.F.; Lei, Q.; Hong, S.; Qiu, W.X.; Liu, L.H.; Cheng, S.X.; Zhang, X.Z. Overcoming the heat endurance of tumor cells by interfering with the anaerobic glycolysis metabolism for improved photothermal therapy. ACS Nano 2017, 11, 1419–1431. [Google Scholar] [CrossRef]
- Sheng, D.; Liu, T.; Deng, L.; Zhang, L.; Li, X.; Xu, J.; Hao, L.; Li, P.; Ran, H.; Chen, H.; et al. Perfluorooctyl bromide & indocyanine green co-loaded nanoliposomes for enhanced multimodal imaging-guided phototherapy. Biomaterials 2018, 165, 1–13. [Google Scholar]
- Wang, W.; Wang, P.; Tang, X.; Elzatahry, A.A.; Wang, S.; Dahyan, D.A.; Zhao, M.; Yao, C.; Hung, C.; Zhu, X.; et al. Facile synthesis of uniform virus-like mesoporous silica nanoparticles for enhanced cellular internalization. ACS Cent. Sci. 2017, 3, 839–846. [Google Scholar] [CrossRef]
- Yang, R.; Wang, P.; Lou, K.; Dang, Y.; Tian, H.; Li, Y.; Gao, Y.; Huang, W.; Zhang, Y.; Liu, X.; et al. Biodegradable nanoprobe for NIR-II fluorescence image-guided surgery and enhanced breast cancer radiotherapy efficacy. Adv. Sci. 2022, 9, 2104728. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, P.; Xie, F.; Chen, J.; Cai, M.; Li, Y.; Yan, J.; Lin, Q.; Luo, F. Virus-inspired hollow mesoporous gadolinium-bismuth nanotheranostics for magnetic resonance imaging-guided synergistic photodynamic-radiotherapy. Adv. Healthc. Mater. 2022, 11, 2102060. [Google Scholar] [CrossRef]
- Shao, C.; Shen, A.; Zhang, M.; Meng, X.; Song, C.; Liu, Y.; Gao, X.; Wang, P.; Bu, W. Oxygen vacancies enhanced CeO2:Gd nanoparticles for sensing a tumor vascular microenvironment by magnetic resonance imaging. ACS Nano 2018, 12, 12629–12637. [Google Scholar] [CrossRef] [PubMed]
- Tapeinos, C.; Battaglini, M.; Prato, M.; Rosa, G.L.; Scarpellini, A.; Ciofani, G. CeO2 Nanoparticles-loaded pH-responsive microparticles with antitumoral properties as therapeutic modulators for osteosarcoma. ACS Omega 2018, 3, 8952–8962. [Google Scholar] [CrossRef] [PubMed]
- Pugachevskii, M.; Kim, E.S.; Kim, N.Y. Selectively acting agent based on ablated CeO2 nanoparticles for photodynamic therapy of brain cancer. BRO Rep. 2019, 6, S54–S345. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Huang, B.; Zhu, S.W.; Zhou, J.J.; Chen, D.R.; Cui, R.; Zhang, M.; Sun, Z.J. Theranostic near-infrared-IIb emitting nanoprobes for promoting immunogenic radiotherapy and abscopal effects against cancer metastasis. Nat. Commun. 2021, 12, 7149. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.; Li, Y.; Ma, J.; Wang, P.; Chen, Q.; Hu, L. Hollow Mesoporous CeO2-Based Nanoenzymes Fabrication for Effective Synergistic Eradication of Malignant Breast Cancer via Photothermal–Chemodynamic Therapy. Pharmaceutics 2022, 14, 1717. https://doi.org/10.3390/pharmaceutics14081717
Tan H, Li Y, Ma J, Wang P, Chen Q, Hu L. Hollow Mesoporous CeO2-Based Nanoenzymes Fabrication for Effective Synergistic Eradication of Malignant Breast Cancer via Photothermal–Chemodynamic Therapy. Pharmaceutics. 2022; 14(8):1717. https://doi.org/10.3390/pharmaceutics14081717
Chicago/Turabian StyleTan, Huaxin, Yongzhen Li, Jiaying Ma, Peiyuan Wang, Qiaoling Chen, and Lidan Hu. 2022. "Hollow Mesoporous CeO2-Based Nanoenzymes Fabrication for Effective Synergistic Eradication of Malignant Breast Cancer via Photothermal–Chemodynamic Therapy" Pharmaceutics 14, no. 8: 1717. https://doi.org/10.3390/pharmaceutics14081717