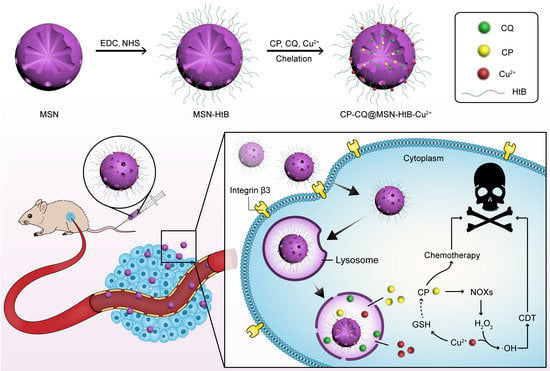
Cu2+-Chelating Mesoporous Silica Nanoparticles for Synergistic Chemotherapy/Chemodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of MSN and MSN−NH2
2.2. Preparation of MSN−HtB
2.3. Preparation of CP/CQ@MSN−HtB/Cu2+
2.4. Materials’ Characterization
DL = (weight of drug loaded in MSNs/total weight of NPs)
2.5. Chelation of Cu2+ with His-Tag
2.6. In Vitro Release Experiments
2.7. Evaluation of Extracellular ·OH Production Capacity
2.8. Cell Culture
2.9. Cytotoxicity Studies
2.10. Cellular Uptake
2.11. Intracellular ROS Generation
2.12. In Vivo Biodistribution
2.13. In Vivo Antitumor Efficacy and Biosafety
2.14. Histological Analysis
2.15. Kaplan–Meier Analysis
2.16. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of CP/CQ@MSN−HtB/Cu2+ NPs
3.2. In Vitro Drug Release
3.3. Evaluation of Extracellular ·OH Production
3.4. Intracellular Uptake
3.5. Intracellular ·OH Detection
3.6. Cytocompatibility and Cytotoxicity
3.7. In Vivo Biodistribution
3.8. In Vivo Antitumor Efficacy and Biosafety
3.9. Histological Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, C.L.; Lu, C.H.; Song, X.Y.; Yang, H.H.; Wang, X.R. Bioresponsive Controlled Release Using Mesoporous Silica Nanoparticles Capped with Aptamer-Based Molecular Gate. J. Am. Chem. Soc. 2011, 133, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Suteewong, T.; Sai, H.; Cohen, R.; Wang, S.; Bradbury, M.; Baird, B.; Gruner, S.M.; Wiesner, U. Highly Aminated Mesoporous Silica Nanoparticles with Cubic Pore Structure. J. Am. Chem. Soc. 2011, 133, 172–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.S.; Haynes, C.L. Synthesis and Characterization of Biocompatible and Size-Tunable Multifunctional Porous Silica Nanoparticles. Chem. Mater. 2009, 21, 3979–3986. [Google Scholar] [CrossRef]
- Corbalan, J.J.; Medina, C.; Jacoby, A.; Malinski, T.; Radomski, M.W. Amorphous Silica Nanoparticles Aggregate Human Platelets: Potential Implications for Vascular Homeostasis. Int. J. Nanomed. 2012, 7, 631–639. [Google Scholar]
- Mal, N.K.; Fujiwara, M.; Tanaka, Y. Photocontrolled Reversible Release of Guest Molecules from Coumarin-Modified Mesoporous Silica. Nature 2003, 421, 350–353. [Google Scholar] [CrossRef]
- Cai, Y.; Deng, T.; Pan, Y.; Zink, J.I. Use of Ferritin Capped Mesoporous Silica Nanoparticles for Redox and pH Triggered Drug Release In Vitro and In Vivo. Adv. Funct. Mater. 2020, 30, 2002043. [Google Scholar] [CrossRef]
- Jia, L.; Li, Q.; Zhang, J.; Huang, L.; Qi, C.; Xu, L.; Liu, X.; Wang, G.; Wang, L.; Wang, Z. Safe and Effective Reversal of Cancer Multidrug Resistance Using Sericin-Coated Mesoporous Silica Nanoparticles for Lysosome-Targeting Delivery in Mice. Small 2017, 13, 1602567. [Google Scholar]
- Xiao, D.; Hu, J.J.; Zhu, J.Y.; Wang, S.B.; Zhuo, R.X.; Zhang, X.Z. A Redox-Responsive Mesoporous Silica Nanoparticle with a Therapeutic Peptide Shell for Tumor Targeting Synergistic Therapy. Nanoscale 2016, 8, 16702–16709. [Google Scholar] [CrossRef]
- Chen, F.; Hong, H.; Zhang, Y.; Valdovinos, H.F.; Shi, S.; Kwon, G.S.; Theuer, C.P.; Barnhart, T.E.; Cai, W. In Vivo Tumor Targeting and Image-Guided Drug Delivery with Antibody-Conjugated, Radiolabeled Mesoporous Silica Nanoparticles. ACS Nano 2013, 7, 9027–9039. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Ma, T.; Tang, W.; Wang, X.; Wang, Y.; Zhuang, J.; Zhu, Y.; Wang, P. Reversibly-Regulated Drug Release Using Poly (tannic acid) Fabricated Nanocarriers for Reduced Secondary Side Effects in Tumor Therapy. Nanoscale Horiz. 2020, 5, 986–998. [Google Scholar] [CrossRef]
- Wu, J.; Williams, G.R.; Niu, S.; Gao, F.; Tang, R.; Zhu, L.M. A Multifunctional Biodegradable Nanocomposite for Cancer Theranostics. Adv. Sci. 2019, 6, 1802001. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, L.L.; Jia, P.; Li, Q.L.; Sun, Y.L.; Zhang, J.; Ning, Y.Q.; Yu, J.; Yang, Y.W. Near-Infrared Light-Responsive Supramolecular Nanovalve Based on Mesoporous Silica-Coated Gold Nanorods. Chem. Sci. 2014, 5, 2804–2808. [Google Scholar] [CrossRef]
- Lai, C.Y.; Trewyn, B.G.; Jeftinija, D.M.; Jeftinija, K.; Xu, S.; Jeftinija, S.; Lin, V.S.Y. A Mesoporous Silica Nanosphere-Based Carrier System with Chemically Removable CdS Nanoparticle Caps for Stimuli-Responsive Controlled Release of Neurotransmitters and Drug Molecules. J. Am. Chem. Soc. 2003, 125, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, W.; Xiao, H.; Chen, Y.; Chen, M.; Li, J. Intelligent Drug Delivery System Based on Mesoporous Silica Nanoparticles Coated with an Ultra-pH-Sensitive Gatekeeper and Poly (ethylene glycol). ACS Macro Lett. 2016, 5, 55–58. [Google Scholar] [CrossRef]
- Lin, J.T.; Liu, Z.K.; Zhu, Q.L.; Rong, X.H.; Liang, C.L.; Wang, J.; Ma, D.; Sun, J.; Wang, G.H. Redox-Responsive Nanocarriers for Drug and Gene co-Delivery Based on Chitosan Derivatives Modified Mesoporous Silica Nanoparticles. Colloids Surf. B 2017, 155, 41–50. [Google Scholar] [CrossRef]
- Patel, K.; Angelos, S.; Dichtel, W.R.; Coskun, A.; Yang, Y.W.; Zink, J.I.; Stoddart, J.F. Enzyme-Responsive Snap-Top Covered Silica Nanocontainers. J. Am. Chem. Soc. 2008, 130, 2382–2383. [Google Scholar] [CrossRef] [Green Version]
- Lei, Q.; Qiu, W.X.; Hu, J.J.; Cao, P.X.; Zhu, C.H.; Cheng, H.; Zhang, X.Z. Multifunctional Mesoporous Silica Nanoparticles with Thermal-Responsive Gatekeeper for NIR Light-Triggered Chemo/Photothermal-Therapy. Small 2016, 12, 4286–4298. [Google Scholar] [CrossRef]
- Cho, I.H.; Shim, M.K.; Jung, B.; Jang, E.H.; Park, M.J.; Kang, H.C.; Kim, J.H. Heat Shock Responsive Drug Delivery System Based on Mesoporous Silica Nanoparticles Coated with Temperature Sensitive Gatekeeper. Micropor. Mesopor. Mater. 2017, 253, 96–101. [Google Scholar] [CrossRef]
- Chen, W.; Cheng, C.A.; Zink, J.I. Spatial, Temporal, and Dose Control of Drug Delivery Using Noninvasive Magnetic Stimulation. ACS Nano 2019, 13, 1292–1308. [Google Scholar] [CrossRef]
- Wu, J.; Bremner, D.H.; Niu, S.; Wu, H.; Wu, J.; Wang, H.; Li, H.; Zhu, L.M. Functionalized MoS2 Nanosheet-Capped Periodic Mesoporous Organosilicas as a Multifunctional Platform for Synergistic Targeted Chemo-Photothermal Therapy. Chem. Eng. J. 2018, 342, 90–102. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.J.; Zhang, A.Q.; Hu, J.J.; He, F.; Zeng, X.; Zhang, X.Z. Multifunctional Peptide-Amphiphile End-Capped Mesoporous Silica Nanoparticles for Tumor Targeting Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Oh, E.-T.; Yoon, H.; Kim, H.; Park, H.J.; Kim, C. Mesoporous Nanocontainer Gated by Stimuli-Responsive Peptide for Selective Triggering of Intracellular Drug Release. Nanoscale 2016, 8, 8070–8077. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liang, P.; Tang, Q.; Yang, X.; Si, W.; Huang, W.; Zhang, Q.; Dong, X. Diketopyrrolopyrrole-Triphenylamine Organic Nanoparticles as Multifunctional Reagents for Photoacoustic Imaging-Guided Photodynamic/Photothermal Synergistic Tumor Therapy. ACS Nano 2017, 11, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Zhu, M.; Lin, G.; Liu, J.; Zhou, Z.; Tian, X.; Pan, Y. PEGylated Au@Pt Nanodendrites as Novel Theranostic Agents for Computed Tomography Imaging and Photothermal/Radiation Synergistic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Li, J.; Mohammed, F.; Wang, Y.; Tou, K.; Liu, X.; Wen, P.; Kinoh, H.; Anraku, Y.; Chen, H.; et al. Therapeutic Polymersome Nanoreactors with Tumor-Specific Activable Cascade Reactions for Cooperative Cancer Therapy. ACS Nano 2019, 13, 2357–2369. [Google Scholar] [CrossRef]
- Li, L.; Yang, Z.; Fan, W.; He, L.; Cui, C.; Zou, J.; Tang, W.; Jacobson, O.; Wang, Z.; Niu, G.; et al. In Situ Polymerized Hollow Mesoporous Organosilica Biocatalysis Nanoreactor for Enhancing ROS-Mediated Anticancer Therapy. Adv. Funct. Mater. 2020, 30, 1907716. [Google Scholar] [CrossRef]
- Xue, T.; Xu, C.; Wang, Y.; Wang, Y.; Tian, H.; Zhang, Y. Doxorubicin-Loaded Nanoscale Metal-Organic Framework for Tumor-Targeting Combined Chemotherapy and Chemodynamic Therapy. Biomater. Sci. 2019, 7, 4615–4623. [Google Scholar] [CrossRef]
- Shen, Z.; Song, J.; Yung, B.C.; Zhou, Z.; Wu, A.; Chen, X. Emerging Strategies of Cancer Therapy Based on Ferroptosis. Adv. Mater. 2018, 30, 1704007. [Google Scholar] [CrossRef]
- Wang, S.; Li, F.; Qiao, R.; Hu, X.; Liao, H.; Chen, L.; Wu, J.; Wu, H.; Zhao, M.; Liu, J.; et al. Arginine-Rich Manganese Silicate Nanobubbles as a Ferroptosis-Inducing Agent for Tumor-Targeted Theranostics. ACS Nano 2018, 12, 12380–12392. [Google Scholar] [CrossRef]
- Hao, Y.N.; Zhang, W.X.; Gao, Y.R.; Wei, Y.N.; Shu, Y.; Wang, J.H. State-of-the-Art Advances of Copper-Based Nanostructures in the Enhancement of Chemodynamic Therapy. J. Mater. Chem. B 2021, 9, 250–266. [Google Scholar] [CrossRef]
- Duan, X.; He, C.; Kron, S.J.; Lin, W. Nanoparticle Formulations of Cisplatin for Cancer Therapy. WIREs Nanomed. Nanobiotechnol. 2016, 8, 776–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Lee, J.H.; Kim, S.J.; Oh, G.S.; Moon, H.D.; Kwon, K.B.; Park, C.; Park, B.H.; Lee, H.K.; Chung, S.Y.; et al. Roles of NADPH Oxidases in Cisplatin-Induced Reactive Oxygen Species Generation and Ototoxicity. J. Neurosci. 2010, 30, 3933–3946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, P.; Xiao, H.; Yu, C.; Liu, J.; Cheng, Z.; Song, H.; Zhang, X.; Li, C.; Wang, J.; Gu, Z.; et al. Enhanced Cisplatin Chemotherapy by Iron Oxide Nanocarrier-Mediated Generation of Highly Toxic Reactive Oxygen Species. Nano Lett. 2017, 17, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Lyu, X.; Wang, Z.; Jin, H.; Lu, S.; Xing, D.; Hu, X. Cocktail Polyprodrug Nanoparticles Concurrently Release Cisplatin and Peroxynitrite-Generating Nitric Oxide in Cisplatin-Resistant Cancers. Chem. Eng. J. 2020, 402, 126125. [Google Scholar] [CrossRef]
- Marques, M.P.M.; Gianolio, D.; Cibin, G.; Tomkinson, J.; Parker, S.F.; Valero, R.; Lopes, R.P.; Carvalho, L.A.E.B. A Molecular View of Cisplatin’s Mode of Action: Interplay with DNA Bases and Acquired Resistance. Phys. Chem. Chem. Phys. 2015, 17, 5155–5171. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Tung, C.H. Redox-Responsive Cisplatin Nanogels for Anticancer Drug Delivery. Chem. Commun. 2018, 54, 8367–8370. [Google Scholar] [CrossRef]
- Xiang, H.; You, C.; Liu, W.; Wang, D.; Chen, Y.; Dong, C. Chemotherapy-Enabled/Augmented Cascade Catalytic Tumor-Oxidative Nanotherapy. Biomaterials 2021, 277, 121071. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, P.; Li, S.; Fu, C.; Ding, C. Aggregation-Disaggregation-Switched Sensing Strategy for Copper(II) Ion and Histidine in Aqueous Solution and Living Cell Imaging. Dye. Pigment. 2019, 171, 107697. [Google Scholar] [CrossRef]
- Eldin, M.M.; Rahman, S.A.; Fawal, G.F.E. Novel Immobilized Cu2+-Aminated Poly (methyl methacrylate) Grafted Cellophane Membranes for Affinity Separation of His-Tag Chitinase. Polym. Bull. 2020, 77, 135–151. [Google Scholar] [CrossRef]
- Ueda, E.K.M.; Gout, P.W.; Morganti, L. Current and Prospective Applications of Metal Ion-Protein Binding. J. Chromatogr. A 2003, 988, 1–23. [Google Scholar] [CrossRef]
- Chen, Q.; Manning, C.D.; Millar, H.; McCabe, F.L.; Ferrante, C.; Sharp, C.; Arruda, L.S.; Doshi, P.; Nakada, M.T.; Anderson, G.M. CNTO 95, a Fully Human Anti αv Integrin Antibody, Inhibits Cell Signaling, Migration, Invasion, and Spontaneous Metastasis of Human Breast Cancer Cells. Clin. Exp. Metastasis 2008, 25, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bisanz, K.; Yu, J.; Edlund, M.; Spohn, B.; Hung, M.C.; Chung, L.W.K.; Hsieh, C.L. Targeting ECM-Integrin Interaction with Liposome-Encapsulated Small Interfering RNAs Inhibits the Growth of Human Prostate Cancer in a Bone Xenograft Imaging Model. Mol. Ther. 2005, 12, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.R.; Chay, C.H.; Pienta, K.J. The Role of αvβ3 in Prostate Cancer Progression. Neoplasia 2002, 4, 191–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mas-Moruno, C.; Beck, J.G.; Doedens, L.; Frank, A.O.; Marinelli, L.; Cosconati, S.; Novellino, E.; Kessler, H. Increasing αvβ3 Selectivity of the Anti-Angiogenic Drug Cilengitide by N-Methylation. Angew. Chem. Int. Ed. 2011, 50, 9496–9500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Shan, X.; Meng, X.; Gu, T.; Lu, Q.; Zhang, J.; Chen, J.; Jiang, Q.; Ning, X. The First Integrins β3-Mediated Cellular and Nuclear Targeting Therapeutics for Prostate Cancer. Biomaterials 2019, 223, 119471. [Google Scholar] [CrossRef]
- Zhang, W.; Tung, C.H. Lysosome Enlargement Enhanced Photochemotherapy Using a Multifunctional Nanogel. ACS Appl. Mater. Interfaces 2018, 10, 4343–4348. [Google Scholar] [CrossRef]
- Zhang, W.; Zhe, Z.; Tung, C.H. Beyond Chemotherapeutics: Cisplatin as a Temporary Buckle to Fabricate Drug-Loaded Nanogels. Chem. Commun. 2017, 53, 779–782. [Google Scholar] [CrossRef]
- Parasuraman, P.; Antony, A.P.; Sharan, A.; Siddhardha, B.; Kasinathan, K.; Bahkali, N.A.; Dawoud, T.M.; Syed, A. Antimicrobial photodynamic activity of toluidine blue encapsulated in mesoporous silica nanoparticles against Pseudomonas aeruginosa and Staphylococcus aureus. Biofouling 2019, 35, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Anju, V.T.; Paramanantham, P.; Sruthil Lal, S.B.; Sharan, A.; Syed, A.; Bahkali, N.A.; Alsaedi, M.H.; Kaviyarasu, K.; Busi, S. Antimicrobial photodynamic activity of toluidine blue-carbon nanotube conjugate against Pseudomonas aeruginosa and Staphylococcus aureus—Understanding the mechanism of action. Photodiagn. Photodyn. Ther. 2019, 27, 305–316. [Google Scholar]
- Parasuraman, P.; Anju, V.T.; Lal, S.B.; Sharan, A.; Busi, S.; Kaviyarasu, K.; Arshad, M.; Dawoud, T.M.S.; Syed, A. Synthesis and antimicrobial photodynamic effect of methylene blue conjugated carbon nanotubes on E. coli and S. aureus. Photochem. Photobiol. Sci. 2019, 18, 563–576. [Google Scholar] [CrossRef]
- Yuan, P.X.; Deng, S.Y.; Zheng, C.Y.; Cosnier, S.; Shan, D. In Situ Formed Copper Nanoparticles Templated by TdT-Mediated DNA for Enhanced SPR Sensor-Based DNA Assay. Biosens. Bioelectron. 2017, 97, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Li, X.; Ye, S.; Yin, G.; Zeng, Q. Catalyzed Oxidative Degradation of Methylene Blue by in Situ Generated Cobalt (II)-Bicarbonate Complexes with Hydrogen Peroxide. Appl. Catal. B 2011, 102, 37–43. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Guo, L.; He, Q.; Chen, F.; Zhou, J.; Feng, J.; Shi, J. Hollow/Rattle-Type Mesoporous Nanostructures by a Structural Difference Based Selective Etching Strategy. ACS Nano 2010, 4, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, E. Immobilized Metal-ion Affinity Chromatography: Imidazole Proton Pump and Chromatographic Sequelae. I. Proton Pump. J. Mol. Recogn. 1996, 9, 389. [Google Scholar] [CrossRef]
- Liu, D.; Liu, M.; Wan, Y.; Zhou, X.; Yang, S.; An, L.; Huang, G.; Tian, Q. Remodeling endogenous H2S microenvironment in colon cancer to enhance chemodynamic therapy. Chem. Eng. J. 2021, 422, 130098. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, Y.; Chen, M.; Chen, J.; Zeng, Z.; Xu, X.; Huang, B.; Luo, Y.; Xiao, Z.; Ding, Y.; et al. Bone-targeted oxidative stress nanoamplifier for synergetic chemo/chemodynamic therapy of bone metastases through increasing generation and reducing elimination of ROS. Chem. Eng. J. 2020, 399, 125667. [Google Scholar] [CrossRef]
- Hao, Y.; Gao, Y.; Fan, Y.; Zhang, C.; Zhan, M.; Cao, X.; Shi, X.; Guo, R. A tumor microenvironment-responsive poly (amidoamine) dendrimer nanoplatform for hypoxia-responsive chemo/chemodynamic therapy. J. Nanobiotechnol. 2022, 20, 43. [Google Scholar] [CrossRef]
- Ren, Z.; Sun, S.; Sun, R.; Cui, G.; Hong, L.; Rao, B.; Li, A.; Yu, Z.; Kan, Q.; Mao, Z. A Metal-Ppolyphenol-Coordinated Nanomedicine for Synergistic Cascade Cancer Chemotherapy and Chemodynamic Therapy. Adv. Mater. 2020, 32, 1906024. [Google Scholar] [CrossRef]
- Ji, X.; Kong, N.; Wang, J.; Li, W.; Xiao, Y.; Gan, S.T.; Zhang, Y.; Li, Y.; Song, X.; Xiong, Q.; et al. A Novel Top-Down Synthesis of Ultrathin 2D Boron Nanosheets for Multimodal Imaging-Guided Cancer Therapy. Adv. Mater. 2018, 30, 1803031. [Google Scholar] [CrossRef]
- Vaghasiya, K.; Ray, E.; Sharma, A.; Katare, O.P.; Verma, R.K. Matrix Metalloproteinase-Responsive Mesoporous Silica Nanoparticles Cloaked with Cleavable Protein for “Self-Actuating” On-Demand Controlled Drug Delivery for Cancer Therapy. ACS Appl. Bio Mater. 2020, 3, 4987–4999. [Google Scholar] [CrossRef]
- Huang, L.; Liu, M.; Mao, L.; Xu, D.; Wan, Q.; Zeng, G.; Shi, Y.; Wen, Y.; Zhang, X.; Wei, Y. Preparation and Controlled Drug Delivery Applications of Mesoporous Silica Polymer Nanocomposites Through the Visible Light Induced Surface-Initiated ATRP. Appl. Surf. Sci. 2017, 412, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Wu, Y.; Ye, H.; Yu, S.; He, C.; Chen, X. Interleukin-15 and Cisplatin Co-Encapsulated Thermosensitive Polypeptide Hydrogels for Combined Immuno-Chemotherapy. J. Control. Release 2017, 255, 81–93. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lou, J.; Williams, G.R.; Ye, Y.; Ren, D.; Shi, A.; Wu, J.; Chen, W.; Zhu, L.-M. Cu2+-Chelating Mesoporous Silica Nanoparticles for Synergistic Chemotherapy/Chemodynamic Therapy. Pharmaceutics 2022, 14, 1200. https://doi.org/10.3390/pharmaceutics14061200
Zhang Y, Lou J, Williams GR, Ye Y, Ren D, Shi A, Wu J, Chen W, Zhu L-M. Cu2+-Chelating Mesoporous Silica Nanoparticles for Synergistic Chemotherapy/Chemodynamic Therapy. Pharmaceutics. 2022; 14(6):1200. https://doi.org/10.3390/pharmaceutics14061200
Chicago/Turabian StyleZhang, Yanyan, Jiadong Lou, Gareth R. Williams, Yuhan Ye, Dandan Ren, Anhua Shi, Junzi Wu, Wenling Chen, and Li-Min Zhu. 2022. "Cu2+-Chelating Mesoporous Silica Nanoparticles for Synergistic Chemotherapy/Chemodynamic Therapy" Pharmaceutics 14, no. 6: 1200. https://doi.org/10.3390/pharmaceutics14061200