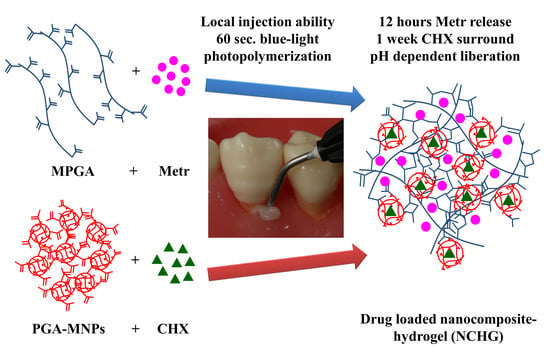
Combined Release of Antiseptic and Antibiotic Drugs from Visible Light Polymerized Biodegradable Nanocomposite Hydrogels for Periodontitis Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Modifications of PGA
2.2. Characterization of the Methacrylation Reaction
2.3. Characterization of the PGA-MNPs
2.4. Synthesis of NCHGs
2.5. Characterization of the MPGA/PGA-MNP NCHG
2.5.1. Mechanical Investigations
2.5.2. Swelling Properties
2.5.3. Study of Drug Release Properties on Different pH
2.5.4. Cell Viability Assay
2.5.5. Vitality Staining
2.5.6. Antibiotic Release Examination in Agar Plates
2.5.7. Time–Kill Experiments
2.6. Statistical Analysis
3. Results
3.1. Modifications of PGA
3.2. NCHG Preparation
3.3. Characterization of the MPGA/PGA-MNP NCHGs
3.3.1. Mechanical Investigations
3.3.2. Swelling Properties
3.3.3. Study of Drug Release Properties on Different pH
3.3.4. Cell Viability Assay
3.3.5. Viability Staining on the Hydrogel Surface
3.3.6. Antibiotic Effect Investigation
3.3.7. Time–Kill Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emani, S.; Gunjiganur, G.; Mehta, D. Determination of the antibacterial activity of simvastatin against periodontal pathogens, Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans: An in vitro study. Contemp. Clin. Dent. 2014, 5, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Nastri, L.; De Rosa, A.; De Gregorio, V.; Grassia, V.; Donnarumma, G. A New Controlled-Release Material Containing Metronidazole and Doxycycline for the Treatment of Periodontal and Peri-Implant Diseases: Formulation and In Vitro Testing. Int. J. Dent. 2019, 2019, 9374607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albuquerque, M.T.P.; Nagata, J.; Bottino, M.C. Antimicrobial Efficacy of Triple Antibiotic-eluting Polymer Nanofibers against Multispecies Biofilm. J. Endod. 2017, 43, S51–S56. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Slots, J. Local delivery of antimicrobial agents in the periodontal pocket. Periodontol. 2000 1996, 10, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Chotitumnavee, J.; Parakaw, T.; Srisatjaluk, R.L.; Pruksaniyom, C.; Pisitpipattana, S.; Thanathipanont, C.; Amarasingh, T.; Tiankhum, N.; Chimchawee, N.; Ruangsawasdi, N. In vitro evaluation of local antibiotic delivery via fibrin hydrogel. J. Dent. Sci. 2019, 14, 7–14. [Google Scholar] [CrossRef]
- H.R, R.; Dhamecha, D.; Jagwani, S.; Rao, M.; Jadhav, K.; Shaikh, S.; Puzhankara, L.; Jalalpure, S. Local drug delivery systems in the management of periodontitis: A scientific review. J. Control. Release 2019, 307, 393–409. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, S.; Zhang, S.; Dong, S.; Hu, J.; Kang, L.; Yang, X. A double-layer hydrogel based on alginate-carboxymethyl cellulose and synthetic polymer as sustained drug delivery system. Sci. Rep. 2021, 11, 9142. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontol. 2000 2020, 84, 14–34. [Google Scholar] [CrossRef]
- Slots, J. Primer on etiology and treatment of progressive/severe periodontitis: A systemic health perspective. Periodontol. 2000 2020, 83, 272–276. [Google Scholar] [CrossRef]
- Johnson, A.; Kong, F.; Miao, S.; Lin, H.-T.V.; Thomas, S.; Huang, Y.-C.; Kong, Z.-L. Therapeutic effects of antibiotics loaded cellulose nanofiber and κ-carrageenan oligosaccharide composite hydrogels for periodontitis treatment. Sci. Rep. 2020, 10, 18037. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Sagar, S.V.; Daisy, H. Clinical and Microbiologic Effects of Subgingivally Delivered 0.5% Azithromycin in the Treatment of Chronic Periodontitis. J. Periodontol. 2008, 79, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Jang, W.J.; Park, S.H.; Kong, I.-S. Antioxidant and gastrointestinal cytoprotective effect of edible polypeptide poly-γ-glutamic acid. Int. J. Biol. Macromol. 2020, 153, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Bakó, J.; Kerényi, F.; Hrubi, E.; Varga, I.; Daróczi, L.; Dienes, B.; Csernoch, L.; Gáll, J.; Hegedűs, C. Poly-γ-Glutamic Acid Nanoparticles Based Visible Light-Curable Hydrogel for Biomedical Application. J. Nanomater. 2016, 2016, 7350516. [Google Scholar] [CrossRef] [Green Version]
- Bakó, J.; Vecsernyés, M.; Ujhelyi, Z.; Kovácsné, I.B.; Borbíró, I.; Bíró, T.; Borbély, J.; Hegedűs, C. Composition and characterization of in situ usable light cured dental drug delivery hydrogel system. J. Mater. Sci. Mater. Med. 2013, 24, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M. Antibiotics in the treatment of periodontitis. Dent. Update 2004, 31, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Pantlin, L. Is there a Role for Antibiotics in Periodontal Treatment? Dent. Update 2008, 35, 493–496. [Google Scholar] [CrossRef]
- Da Rocha, H.A.J.; Silva, C.F.; Santiago, F.L.; Martins, L.G.; Dias, P.C.; De Magalhães, D. Local Drug Delivery Systems in the Treatment of Periodontitis: A Literature Review. J. Int. Acad. Periodontol. 2015, 17, 82–90. [Google Scholar]
- Rafieian, S.; Mirzadeh, H.; Mahdavi, H.; Masoumi, M.E. A review on nanocomposite hydrogels and their biomedical applications. Sci. Eng. Compos. Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Farjadian, F.; Rezaeifard, S.; Naeimi, M.; Ghasemi, S.; Mohammadi-Samani, S.; Welland, M.E.; Tayebi, L. Temperature and pH-responsive nano-hydrogel drug delivery system based on lysine-modified poly (vinylcaprolactam). Int. J. Nanomed. 2019, 14, 6901–6915. [Google Scholar] [CrossRef] [Green Version]
- Ainamo, J.; Lie, T.; Ellingsen, B.H.; Hansen, B.F.; Johansson, L.-Å.; Karring, T.; Kisch, J.; Paunio, K.; Stoltze, K. Clinical responses to subgingival application of a metronidazole 25% gel compared to the effect of subgingival scaling in adult periodontitis. J. Clin. Periodontol. 1992, 19, 723–729. [Google Scholar] [CrossRef]
- Loesche, W.J.; Schmidt, E.; Smith, B.A.; Morrison, E.C.; Caffesse, R.; Hujoel, P.P. Effects of Metronidazole on Periodontal Treatment Needs. J. Periodontol. 1991, 62, 247–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musial, W.; Voncina, B.; Pluta, J.; Kokol, V. The Study of Release of Chlorhexidine from Preparations with Modified Thermosensitive Poly-N-isopropylacrylamide Microspheres. Sci. World J. 2012, 2012, 243707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albayaty, Y.N.; Thomas, N.; Jambhrunkar, M.; Al-Hawwas, M.; Kral, A.; Thorn, C.R.; Prestidge, C.A. Enzyme responsive copolymer micelles enhance the anti-biofilm efficacy of the antiseptic chlorhexidine. Int. J. Pharm. 2019, 566, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Hu, W.-K.; Li, H.; Jing, Y.-H.; Kang, H.; Jiang, Q.; Zhang, C. Preparation and characterization of Poly (γ-glutamic acid) hydrogels as potential tissue engineering scaffolds. Chin. J. Polym. Sci. 2014, 32, 1507–1514. [Google Scholar] [CrossRef]
- Tronci, G.; Grant, C.A.; Thomson, N.H.; Russell, S.J.; Wood, D.J. Multi-scale mechanical characterization of highly swollen photo-activated collagen hydrogels. J. R. Soc. Interface 2015, 12, 20141079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wei, X.; Ji, Y.; Yin, L.; Dong, Z.; Chen, F.; Zhong, M.; Shen, J.; Liu, Z.; Chang, L. Adjustable and ultrafast light-cured hyaluronic acid hydrogel: Promoting biocompatibility and cell growth. J. Mater. Chem. B 2020, 8, 5441–5450. [Google Scholar] [CrossRef]
- Greenstein, G. Local Drug Delivery in the Treatment of Periodontal Diseases: Assessing the Clinical Significance of the Results. J. Periodontol. 2006, 77, 565–578. [Google Scholar] [CrossRef]
- Budai-Szucs, M.; Ruggeri, M.; Faccendini, A.; Leber, A.; Rossi, S.; Varga, G.; Bonferoni, M.C.; Valyi, P.; Burian, K.; Csanyi, E.; et al. Electrospun Scaffolds in Periodontal Wound Healing. Polymers 2021, 13, 307. [Google Scholar] [CrossRef]
- Killoy, W.J. The use of locally-delivered chlorhexidine in the treatment of periodontitis. Clinical results. J. Clin. Periodontol. 1998, 25, 953–958. [Google Scholar] [CrossRef]
- Jepsen, K.; Jepsen, S. Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol. 2000 2016, 71, 82–112. [Google Scholar] [CrossRef]
- Lang, N.P.; Catalanotto, F.A.; Knöpfli, R.U.; Antczak, A.A.A. Quality-specific taste impairment following the application of chlorhexidine digluconate mouthrinses. J. Clin. Periodontol. 1988, 15, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Gendron, R.; Grenier, D.; Sorsa, T.; Mayrand, D. Inhibition of the Activities of Matrix Metalloproteinases 2, 8, and 9 by Chlorhexidine. Clin. Diagn. Lab. Immunol. 1999, 6, 437–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grenier, D. Effect of chlorhexidine on the adherence properties of Porphyromonas gingivalis. J. Clin. Periodontol. 1996, 23, 140–142. [Google Scholar] [CrossRef]
- Pietruska, M.; Paniczko, A.; Waszkiel, D.; Pietruski, J.; Bernaczyk, A. Efficacy of local treatment with chlorhexidine gluconate drugs on the clinical status of periodontium in chronic periodontitis patients. Adv. Med. Sci. 2006, 51, 162–165. [Google Scholar] [PubMed]
- Phaechamud, T.; Setthajindalert, O. Cholesterol in situ forming gel loaded with doxycycline hyclate for intra-periodontal pocket delivery. Eur. J. Pharm. Sci. 2017, 99, 258–265. [Google Scholar] [CrossRef]
- Purucker, P.; Mertes, H.; Goodson, J.M.; Bernimoulin, J.-P. Local Versus Systemic Adjunctive Antibiotic Therapy in 28 Patients With Generalized Aggressive Periodontitis. J. Periodontol. 2001, 72, 1241–1245. [Google Scholar] [CrossRef]
- Tan, O.L.; Safii, S.H.; Razali, M. Commercial Local Pharmacotherapeutics and Adjunctive Agents for Nonsurgical Treatment of Periodontitis: A Contemporary Review of Clinical Efficacies and Challenges. Antibiotics 2020, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, D.; Friedman, M. Sustained-release delivery of antimicrobial drugs for the treatment of periodontal diseases: Fantasy or already reality? Periodontol. 2000 2020, 84, 176–187. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Gad, S.F.; Chobisa, D.; Li, Y.; Yeo, Y. Local drug delivery systems for inflammatory diseases: Status quo, challenges, and opportunities. J. Control. Release 2021, 330, 438–460. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Grinberg, V.Y.; Burova, T.V.; Concheiro, A. Stimuli-sensitive cross-linked hydrogels as drug delivery systems: Impact of the drug on the responsiveness. Int. J. Pharm. 2020, 579, 119157. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Shao, J.; Kouwer, P.H.J.; Bronkhorst, E.M.; Jansen, J.A.; Walboomers, X.F.; Yang, F. A tunable and injectable local drug delivery system for personalized periodontal application. J. Control. Release 2020, 324, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Romero, G.; Zambrano-Zaragoza, M.L.; Martínez-Acevedo, L.; Leyva-Gómez, G.; Mendoza-Elvira, S.E.; Quintanar-Guerrero, D. Design and Evaluation of pH-Dependent Nanosystems Based on Cellulose Acetate Phthalate, Nanoparticles Loaded with Chlorhexidine for Periodontal Treatment. Pharmaceutics 2019, 11, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Aati, S.; Ngo, H.; Fawzy, A. pH-dependent delivery of chlorhexidine from PGA grafted mesoporous silica nanoparticles at resin-dentin interface. J. Nanobiotechnol. 2021, 19, 43. [Google Scholar] [CrossRef]
- Fullriede, H.; Abendroth, P.; Ehlert, N.; Doll, K.; Schäske, J.; Winkel, A.; Stumpp, S.N.; Stiesch, M.; Behrens, P. pH-responsive release of chlorhexidine from modified nanoporous silica nanoparticles for dental applications. BioNanoMaterials 2016, 17, 59–72. [Google Scholar] [CrossRef]
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q.; et al. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef]
- Batool, F.; Agossa, K.; Lizambard, M.; Petit, C.; Bugueno, I.M.; Delcourt-Debruyne, E.; Benkirane-Jessel, N.; Tenenbaum, H.; Siepmann, J.; Siepmann, F.; et al. In-situ forming implants loaded with chlorhexidine and ibuprofen for periodontal treatment: Proof of concept study in vivo. Int. J. Pharm. 2019, 569, 118564. [Google Scholar] [CrossRef]















| n = min. 9 | Control (NCHG) | NCHG + CHX + Metr | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Young modulus (MPa) | 2.3845 | 0.3594 | 0.2237 | 0.0757 |
| Compressive stress (MPa) | 0.2924 | 0.0178 | 0.1094 | 0.0340 |
| Compressive load (N) | 4.8806 | 0.1300 | 1.8458 | 0.6466 |
| Compressive strain (mm/mm) | 0.2224 | 0.0251 | 0.5800 | 0.0733 |
| n = 5 | 4 h | 24 h | ||
|---|---|---|---|---|
| (mm) | Mean | SD | Mean | SD |
| + | 12.5349 | 0.6613 | 14.6773 | 0.9291 |
| NCHG | 10.7816 | 1.3406 | 12.3976 | 0.8360 |
| NCHG + pH 2 | 24.2077 | 3.1961 | 23.1351 | 2.8713 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bako, J.; Toth, F.; Gall, J.; Kovacs, R.; Csík, A.; Varga, I.; Sculean, A.; Zelko, R.; Hegedus, C. Combined Release of Antiseptic and Antibiotic Drugs from Visible Light Polymerized Biodegradable Nanocomposite Hydrogels for Periodontitis Treatment. Pharmaceutics 2022, 14, 957. https://doi.org/10.3390/pharmaceutics14050957
Bako J, Toth F, Gall J, Kovacs R, Csík A, Varga I, Sculean A, Zelko R, Hegedus C. Combined Release of Antiseptic and Antibiotic Drugs from Visible Light Polymerized Biodegradable Nanocomposite Hydrogels for Periodontitis Treatment. Pharmaceutics. 2022; 14(5):957. https://doi.org/10.3390/pharmaceutics14050957
Chicago/Turabian StyleBako, Jozsef, Ferenc Toth, Jozsef Gall, Renato Kovacs, Attila Csík, Istvan Varga, Anton Sculean, Romana Zelko, and Csaba Hegedus. 2022. "Combined Release of Antiseptic and Antibiotic Drugs from Visible Light Polymerized Biodegradable Nanocomposite Hydrogels for Periodontitis Treatment" Pharmaceutics 14, no. 5: 957. https://doi.org/10.3390/pharmaceutics14050957