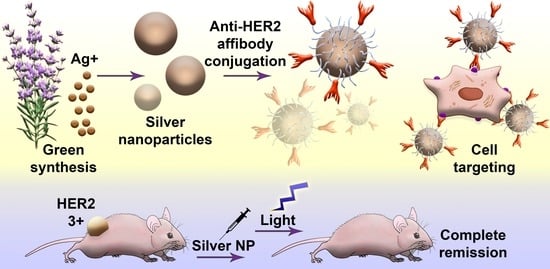
Photothermal Therapy with HER2-Targeted Silver Nanoparticles Leading to Cancer Remission
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
- (i)
- Synthesis, characterization, and modification of silver nanoparticles. First, the optimal conditions for the NP synthesis were found to obtain stable particles in the sub100 nm size range. Then, physicochemical, optical, and photothermal properties of NPs were studied. To introduce cancer cell-targeting capabilities, particles were equipped with anti-HER2 scaffold protein, namely affibody ZHER2:342, through intermediate PEG modification.
- (ii)
- Study of nanoparticle–cell interaction, cytotoxic properties in vitro, and antitumor activity in vivo. First, the processes of interaction of NPs with HER2-overexpressing cancer cells were studied, namely the NP internalization rate and the cytotoxic properties. Next, the light-induced hyperthermia of cancer cells in vitro was demonstrated. Last, the anticancer activity of NPs in vivo using the xenograft HER2-overexpressing tumors was studied. Cancer remission was shown when mice were treated with injections of targeted Ag NPs followed by light irradiation.
2.2. Materials
2.3. Nanoparticle Synthesis
2.4. Scanning Electron Microscopy
2.5. Hyperthermic Properties Study
2.6. Affibody ZHER2:342 Isolation and Purification
2.7. Nanoparticle Modification: BSA Stabilization, PEG Modification, and Affibody Conjugation
2.8. Cell Culture
2.9. FITC and DyLight650 Conjugation
2.10. Confocal Microscopy
2.11. Flow Cytometry
2.12. Viability Assay
2.13. Clonogenic Assay
2.14. ROS Generation Study
2.15. Tumor-Bearing Mice
2.16. In Vivo Therapy
2.17. In Vivo Toxicity
2.18. In Vivo Bioimaging
3. Results
3.1. Synthesis and Characterization of Silver Nanoparticles for HER2-Positive Cancer PTT
3.2. Nanoparticle Stabilization and HER2-Overexpressing Cells Labeling with Affibody ZHER2:342
3.3. Nanoparticle Modification and HER2-Overexpressing Cell Targeting with Ag-PEG-HER2 Nanoparticles
3.4. Imaging Modalities of Ag-PEG-HER2 Nanoparticles
3.5. Cytotoxic Properties of Ag-PEG-HER2 Nanoparticles
3.6. Photothermal Cancer Therapy with Targeted Silver Nanoparticles and External Irradiation on the Xenograft Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, M.-H.; Huang, W.-T.; Satpathy, A.; Su, T.-Y.; Hsiao, M.; Liu, R.-S. Progress and Viewpoints of Multifunctional Composite Nanomaterials for Glioblastoma Theranostics. Pharmaceutics 2022, 14, 456. [Google Scholar] [CrossRef] [PubMed]
- Novoselova, M.V.; German, S.V.; Abakumova, T.O.; Perevoschikov, S.V.; Sergeeva, O.V.; Nesterchuk, M.V.; Efimova, O.I.; Petrov, K.S.; Chernyshev, V.S.; Zatsepin, T.S.; et al. Multifunctional nanostructured drug delivery carriers for cancer therapy: Multimodal imaging and ultrasound-induced drug release. Colloids Surf. B Biointerfaces 2021, 200, 111576. [Google Scholar] [CrossRef] [PubMed]
- Parakhonskiy, B.V.; Shilyagina, N.Y.; Gusliakova, O.I.; Volovetskiy, A.B.; Kostyuk, A.B.; Balalaeva, I.V.; Klapshina, L.G.; Lermontova, S.A.; Tolmachev, V.; Orlova, A.; et al. A method of drug delivery to tumors based on rapidly biodegradable drug-loaded containers. Appl. Mater. Today 2021, 25, 101199. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Shipunova, V.O.; Mirkasymov, A.B.; Nikitin, P.I.; Nikitin, M.P.; Deyev, S.M. Synthesis and Characterization of Hybrid Core-Shell Fe3O4/SiO2 Nanoparticles for Biomedical Applications. Acta Nat. 2017, 9, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Nikitin, M.P.; Shipunova, V.O.; Deyev, S.M.; Nikitin, P.I. Biocomputing based on particle disassembly. Nat. Nanotechnol. 2014, 9, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.; Belmonte-Reche, E.; Gallo, J.; Baltazar, F.; Bañobre-López, M. Magnetic Solid Nanoparticles and Their Counterparts: Recent Advances towards Cancer Theranostics. Pharmaceutics 2022, 14, 506. [Google Scholar] [CrossRef]
- Yashchenok, A.M.; Gusliakova, O.I.; Konovalova, E.V.; Novoselova, M.V.; Shipunova, V.O.; Abakumova, T.O.; Efimova, O.I.; Kholodenko, R.; Schulga, A.A.; Zatsepin, T.S.; et al. Barnase encapsulation into submicron porous CaCO3 particles: Studies of loading and enzyme activity. J. Mater. Chem. B 2021, 9, 8823–8831. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Mashkovich, E.A.; Lipey, N.A.; Popov, A.A.; Shipunova, V.O.; Griaznova, O.Y.; Deryabin, M.S.; Kurin, V.V.; Nikitin, P.I.; Kabashin, A.V.; et al. Direct photoacoustic measurement of silicon nanoparticle degradation promoted by a polymer coating. Chem. Eng. J. 2022, 430, 132860. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Kravets, V.G.; Wu, F.; Imaizumi, S.; Shipunova, V.O.; Deyev, S.M.; Grigorenko, A.N. Phase-Responsive Fourier Nanotransducers for Probing 2D Materials and Functional Interfaces. Adv. Funct. Mater. 2019, 29, 1902692. [Google Scholar] [CrossRef] [Green Version]
- Shipunova, V.O.; Nikitin, M.P.; Zelepukin, I.V.; Nikitin, P.I.; Deyev, S.M.; Petrov, R.V. A comprehensive study of interactions between lectins and glycoproteins for the development of effective theranostic nanoagents. Dokl. Biochem. Biophys. 2015, 464, 315–318. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Popov, A.A.; Shipunova, V.O.; Tikhonowski, G.V.; Mirkasymov, A.B.; Popova-Kuznetsova, E.A.; Klimentov, S.M.; Kabashin, A.V.; Deyev, S.M. Laser-synthesized TiN nanoparticles for biomedical applications: Evaluation of safety, biodistribution and pharmacokinetics. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111717. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.-W. State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-H.; Choi, H.; Kim, J.Y.; Lee, T.-W. Silver-Based Nanoparticles for Surface Plasmon Resonance in Organic Optoelectronics. Part. Part. Syst. Charact. 2015, 32, 164–175. [Google Scholar] [CrossRef]
- Kim, D.; Amatya, R.; Hwang, S.; Lee, S.; Min, K.A.; Shin, M.C. BSA-Silver Nanoparticles: A Potential Multimodal Therapeutics for Conventional and Photothermal Treatment of Skin Cancer. Pharmaceutics 2021, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Boca, S.C.; Potara, M.; Gabudean, A.-M.; Juhem, A.; Baldeck, P.L.; Astilean, S. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Lett. 2011, 311, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Ramírez, O.U.; Valenzuela-Salas, L.M.; Blanco-Salazar, A.; Rodríguez-Arenas, J.A.; Mier-Maldonado, P.A.; García-Ramos, J.C.; Bogdanchikova, N.; Pestryakov, A.; Toledano-Magaña, Y. Antitumor Activity against Human Colorectal Adenocarcinoma of Silver Nanoparticles: Influence of Ag/PVP Ratio. Pharmaceutics 2021, 13, 1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, C.; Yan, X.-P. Fabrication of folate bioconjugated near-infrared fluorescent silver nanoclusters for targeted in vitro and in vivo bioimaging. Chem. Commun. 2014, 50, 14341–14344. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, N.; Qin, Y.; Wu, F.; Xu, Z.; Lan, T.; Cheng, Z.; Zhao, P.; Liu, H. Affibody-functionalized Ag2S quantum dots for photoacoustic imaging of epidermal growth factor receptor overexpressed tumors. Nanoscale 2018, 10, 16581–16590. [Google Scholar] [CrossRef]
- Sun, R.; Zhao, Y.; Wang, Y.; Zhang, Q.; Zhao, P. An affibody-conjugated nanoprobe for IGF-1R targeted cancer fluorescent and photoacoustic dual-modality imaging. Nanotechnology 2021, 32, 205103. [Google Scholar] [CrossRef]
- Park, T.; Lee, S.; Amatya, R.; Cheong, H.; Moon, C.; Kwak, H.D.; Min, K.A.; Shin, M.C. ICG-Loaded PEGylated BSA-Silver Nanoparticles for Effective Photothermal Cancer Therapy. Int. J. Nanomed. 2020, 15, 5459–5471. [Google Scholar] [CrossRef]
- Orlova, A.; Magnusson, M.; Eriksson, T.L.J.; Nilsson, M.; Larsson, B.; Höidén-Guthenberg, I.; Widström, C.; Carlsson, J.; Tolmachev, V.; Ståhl, S.; et al. Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res. 2006, 66, 4339–4348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolmachev, V.; Nilsson, F.Y.; Widström, C.; Andersson, K.; Rosik, D.; Gedda, L.; Wennborg, A.; Orlova, A. 111In-benzyl-DTPA-ZHER2:342, an affibody-based conjugate for in vivo imaging of HER2 expression in malignant tumors. J. Nucl. Med. 2006, 47, 846–853. [Google Scholar] [PubMed]
- Orlov, A.V.; Burenin, A.G.; Shipunova, V.O.; Lizunova, A.A.; Gorshkov, B.G.; Nikitin, P.I. Development of immunoassays using interferometric real-time registration of their kinetics. Acta Nat. 2014, 6, 85–95. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Shramova, E.I.; Schulga, A.A.; Shilova, M.V.; Deyev, S.M.; Proshkina, G.M. Delivery of Barnase to Cells in Liposomes Functionalized by Her2-Specific DARPin Module. Russ. J Bioorg. Chem. 2020, 46, 1156–1161. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Zelepukin, I.V.; Stremovskiy, O.A.; Nikitin, M.P.; Care, A.; Sunna, A.; Zvyagin, A.V.; Deyev, S.M. Versatile Platform for Nanoparticle Surface Bioengineering Based on SiO2-Binding Peptide and Proteinaceous Barnase*Barstar Interface. ACS Appl. Mater. Interfaces 2018, 10, 17437–17447. [Google Scholar] [CrossRef]
- Kotelnikova, P.A.; Shipunova, V.O.; Aghayeva, U.F.; Stremovskiy, O.A.; Nikitin, M.P.; Novikov, I.A.; Schulga, A.A.; Deyev, S.M.; Petrov, R.V. Synthesis of Magnetic Nanoparticles Stabilized by Magnetite-Binding Protein for Targeted Delivery to Cancer Cells. Dokl. Biochem. Biophys. 2018, 481, 198–200. [Google Scholar] [CrossRef]
- Bragina, O.; von Witting, E.; Garousi, J.; Zelchan, R.; Sandström, M.; Orlova, A.; Medvedeva, A.; Doroshenko, A.; Vorobyeva, A.; Lindbo, S.; et al. Phase I Study of 99mTc-ADAPT6, a Scaffold Protein-Based Probe for Visualization of HER2 Expression in Breast Cancer. J. Nucl. Med. 2021, 62, 493–499. [Google Scholar] [CrossRef]
- Sogomonyan, A.S.; Shipunova, V.O.; Soloviev, V.D.; Larionov, V.I. 3D Models of Cellular Spheroids As a Universal Tool for Studying the Cytotoxic Properties of Anticancer Compounds In Vitro. Acta Nat. 2022, 14, 31–39. [Google Scholar] [CrossRef]
- Sörensen, J.; Sandberg, D.; Sandström, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Åström, G.; Lubberink, M.; Garske-Román, U.; Carlsson, J.; et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J. Nucl. Med. 2014, 55, 730–735. [Google Scholar] [CrossRef] [Green Version]
- Belova, M.M.; Shipunova, V.O.; Kotelnikova, P.A.; Babenyshev, A.V.; Rogozhin, E.A.; Cherednichenko, M.Y.; Deyev, S.M. “Green” Synthesis of Cytotoxic Silver Nanoparticles Based on Secondary Metabolites of Lavandula Angustifolia Mill. Acta Nat. 2019, 11, 47–53. [Google Scholar] [CrossRef]
- Peng, S.; McMahon, J.M.; Schatz, G.C.; Gray, S.K.; Sun, Y. Reversing the size-dependence of surface plasmon resonances. Proc. Natl. Acad. Sci. USA 2010, 107, 14530–14534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shipunova, V.O.; Komedchikova, E.N.; Kotelnikova, P.A.; Zelepukin, I.V.; Schulga, A.A.; Proshkina, G.M.; Shramova, E.I.; Kutscher, H.L.; Telegin, G.B.; Kabashin, A.V.; et al. Dual Regioselective Targeting the Same Receptor in Nanoparticle-Mediated Combination Immuno/Chemotherapy for Enhanced Image-Guided Cancer Treatment. ACS Nano 2020, 14, 12781–12795. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wolf, J.K.; Scanlon, M.; Price, J.E.; Hung, M.C. Enhanced c-erbB-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Res. 1993, 53, 891–898. [Google Scholar]
- Soheili, M.; Salami, M. Lavandula angustifolia biological characteristics: An in vitro study. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Centi, S.; Cavigli, L.; Borri, C.; Milanesi, A.; Banchelli, M.; Chioccioli, S.; Khlebtsov, B.N.; Khlebtsov, N.G.; Matteini, P.; Bogani, P.; et al. Small Thiols Stabilize the Shape of Gold Nanorods. J. Phys. Chem. C 2020, 124, 11132–11140. [Google Scholar] [CrossRef]
- Liu, K.; Ohodnicki, P.R.; Kong, X.; Lee, S.S.; Du, H. Plasmonic Au nanorods stabilized within anodic aluminum oxide pore channels against high-temperature treatment. Nanotechnology 2019, 30, 405704. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Wang, G.; Zhang, Q.; Liu, X.; Yin, B.; Fang, D.; Zhao, J.; Zhang, H.; Chen, Y.Q.; Chen, W. Determining antioxidant activities of lactobacilli by cellular antioxidant assay in mammal cells. J. Funct. Foods 2015, 19, 554–562. [Google Scholar] [CrossRef]
- Lee, S.B.; Hassan, M.; Fisher, R.; Chertov, O.; Chernomordik, V.; Kramer-Marek, G.; Gandjbakhche, A.; Capala, J. Affibody molecules for in vivo characterization of HER2-positive tumors by near-infrared imaging. Clin. Cancer Res. 2008, 14, 3840–3849. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Wu, F.-G.; Zhang, X.; Jiang, Y.-W.; Jia, H.-R.; Wang, H.-Y.; Li, Y.-H.; Liu, P.; Gu, N.; Chen, Z. Shape-Dependent Radiosensitization Effect of Gold Nanostructures in Cancer Radiotherapy: Comparison of Gold Nanoparticles, Nanospikes, and Nanorods. ACS Appl. Mater. Interfaces 2017, 9, 13037–13048. [Google Scholar] [CrossRef]
- Rinkenauer, A.C.; Press, A.T.; Raasch, M.; Pietsch, C.; Schweizer, S.; Schwörer, S.; Rudolph, K.L.; Mosig, A.; Bauer, M.; Traeger, A.; et al. Comparison of the uptake of methacrylate-based nanoparticles in static and dynamic in vitro systems as well as in vivo. J. Control. Release 2015, 216, 158–168. [Google Scholar] [CrossRef]
- Zucker, R.M.; Daniel, K.M.; Massaro, E.J.; Karafas, S.J.; Degn, L.L.; Boyes, W.K. Detection of silver nanoparticles in cells by flow cytometry using light scatter and far-red fluorescence. Cytom. A 2013, 83, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Nikitin, M.P.; Nikitin, P.I.; Deyev, S.M. MPQ-cytometry: A magnetism-based method for quantification of nanoparticle-cell interactions. Nanoscale 2016, 8, 12764–12772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Surface-coating-dependent dissolution, aggregation, and reactive oxygen species (ROS) generation of silver nanoparticles under different irradiation conditions. Environ. Sci. Technol. 2013, 47, 10293–10301. [Google Scholar] [CrossRef] [PubMed]
- Shramova, E.; Proshkina, G.; Shipunova, V.; Ryabova, A.; Kamyshinsky, R.; Konevega, A.; Schulga, A.; Konovalova, E.; Telegin, G.; Deyev, S. Dual Targeting of Cancer Cells with DARPin-Based Toxins for Overcoming Tumor Escape. Cancers 2020, 12, 3014. [Google Scholar] [CrossRef]
- Shramova, E.I.; Chumakov, S.P.; Shipunova, V.O.; Ryabova, A.V.; Telegin, G.B.; Kabashin, A.V.; Deyev, S.M.; Proshkina, G.M. Genetically encoded BRET-activated photodynamic therapy for the treatment of deep-seated tumors. Light Sci. Appl. 2022, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Shilova, O.N.; Shramova, E.I.; Deyev, S.M.; Proshkina, G.M. A Highly Specific Substrate for NanoLUC Luciferase Furimazine Is Toxic in vitro and in vivo. Russ. J. Bioorg. Chem. 2018, 44, 225–228. [Google Scholar] [CrossRef]
- Proshkina, G.M.; Shramova, E.I.; Shilova, M.V.; Zelepukin, I.V.; Shipunova, V.O.; Ryabova, A.V.; Deyev, S.M.; Kotlyar, A.B. DARPin_9-29-Targeted Gold Nanorods Selectively Suppress HER2-Positive Tumor Growth in Mice. Cancers 2021, 13, 5235. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Kovalenko, V.L.; Kotelnikova, P.A.; Sogomonyan, A.S.; Shilova, O.N.; Komedchikova, E.N.; Zvyagin, A.V.; Nikitin, M.P.; Deyev, S.M. Targeting Cancer Cell Tight Junctions Enhances PLGA-Based Photothermal Sensitizers’ Performance In Vitro and In Vivo. Pharmaceutics 2021, 14, 43. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Plasmonic photo-thermal therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Shipunova, V.O.; Nikitin, M.P.; Belova, M.M.; Deyev, S.M. Label-free methods of multiparametric surface plasmon resonance and MPQ-cytometry for quantitative real-time measurements of targeted magnetic nanoparticles complexation with living cancer cells. Mater. Today Commun. 2021, 29, 102978. [Google Scholar] [CrossRef]
- Sepúlveda, B.; Angelomé, P.C.; Lechuga, L.M.; Liz-Marzán, L.M. LSPR-based nanobiosensors. Nano Today 2009, 4, 244–251. [Google Scholar] [CrossRef]
- Srituravanich, W.; Fang, N.; Sun, C.; Luo, Q.; Zhang, X. Plasmonic Nanolithography. Nano Lett. 2004, 4, 1085–1088. [Google Scholar] [CrossRef]
- Schröter, U.; Dereux, A. Surface plasmon polaritons on metal cylinders with dielectric core. Phys. Rev. B 2001, 64, 439. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.M.; Langhammer, C.; Zorić, I.; Kasemo, B. Nanoplasmonic probes of catalytic reactions. Science 2009, 326, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-J.; Kim, J.; Chen, H.-Y.; Wu, C.; Dabidian, N.; Sanders, C.E.; Wang, C.-Y.; Lu, M.-Y.; Li, B.-H.; Qiu, X.; et al. Plasmonic nanolaser using epitaxially grown silver film. Science 2012, 337, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Sachan, R.; Ramos, V.; Malasi, A.; Yadavali, S.; Bartley, B.; Garcia, H.; Duscher, G.; Kalyanaraman, R. Oxidation-resistant silver nanostructures for ultrastable plasmonic applications. Adv. Mater. 2013, 25, 2045–2050. [Google Scholar] [CrossRef]
- Baburin, A.S.; Merzlikin, A.M.; Baryshev, A.V.; Ryzhikov, I.A.; Panfilov, Y.V.; Rodionov, I.A. Silver-based plasmonics: Golden material platform and application challenges [Invited]. Opt. Mater. Express 2019, 9, 611. [Google Scholar] [CrossRef]
- Yuan, H.; Khoury, C.G.; Wilson, C.M.; Grant, G.A.; Bennett, A.J.; Vo-Dinh, T. In vivo particle tracking and photothermal ablation using plasmon-resonant gold nanostars. Nanomedicine 2012, 8, 1355–1363. [Google Scholar] [CrossRef] [Green Version]
- Bao, C.; Conde, J.; Pan, F.; Li, C.; Zhang, C.; Tian, F.; Liang, S.; de La Fuente, J.M.; Cui, D. Gold nanoprisms as a hybrid in vivo cancer theranostic platform for in situ photoacoustic imaging, angiography, and localized hyperthermia. Nano Res. 2016, 9, 1043–1056. [Google Scholar] [CrossRef] [Green Version]
- Pitsillides, C.M.; Joe, E.K.; Wei, X.; Anderson, R.R.; Lin, C.P. Selective Cell Targeting with Light-Absorbing Microparticles and Nanoparticles. Biophys. J. 2003, 84, 4023–4032. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, D.P.; Hirsch, L.R.; Halas, N.J.; Payne, J.D.; West, J.L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004, 209, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Norbert, C.C.; Acharyya, R.; Mukherjee, S.; Kathirvel, M.; Patra, C.R. Biosynthesized Silver Nanoparticles for Cancer Therapy and In Vivo Bioimaging. Cancers 2021, 13, 6114. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Y.; Guo, Q.; Wang, Z.; Wang, H.; Yang, Y.; Huang, Y. TAT-modified nanosilver for combating multidrug-resistant cancer. Biomaterials 2012, 33, 6155–6161. [Google Scholar] [CrossRef]
- Behnam, M.A.; Emami, F.; Sobhani, Z.; Koohi-Hosseinabadi, O.; Dehghanian, A.R.; Zebarjad, S.M.; Moghim, M.H.; Oryan, A. Novel Combination of Silver Nanoparticles and Carbon Nanotubes for Plasmonic Photo Thermal Therapy in Melanoma Cancer Model. Adv. Pharm. Bull. 2018, 8, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, B.; Pal, R.; Ali, M.; Singh, L.M.; Shahidur Rahman, D.; Kumar Ghosh, S.; Sengupta, M. Immunomodulatory properties of silver nanoparticles contribute to anticancer strategy for murine fibrosarcoma. Cell. Mol. Immunol. 2016, 13, 191–205. [Google Scholar] [CrossRef]
- Habiba, K.; Aziz, K.; Sanders, K.; Santiago, C.M.; Mahadevan, L.S.K.; Makarov, V.; Weiner, B.R.; Morell, G.; Krishnan, S. Enhancing Colorectal Cancer Radiation Therapy Efficacy using Silver Nanoprisms Decorated with Graphene as Radiosensitizers. Sci. Rep. 2019, 9, 17120. [Google Scholar] [CrossRef]
- Hembram, K.C.; Chatterjee, S.; Sethy, C.; Nayak, D.; Pradhan, R.; Molla, S.; Bindhani, B.K.; Kundu, C.N. Comparative and Mechanistic Study on the Anticancer Activity of Quinacrine-Based Silver and Gold Hybrid Nanoparticles in Head and Neck Cancer. Mol. Pharm. 2019, 16, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Zhang, M.; Cui, H.; Gong, J.; Huang, Y.; Wang, J.; Cui, Y.; Dong, W.; Sun, L.; He, H.; et al. Tat-functionalized Ag-Fe3O4 nano-composites as tissue-penetrating vehicles for tumor magnetic targeting and drug delivery. Acta Pharm. Sin. B 2018, 8, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Swanner, J.; Fahrenholtz, C.D.; Tenvooren, I.; Bernish, B.W.; Sears, J.J.; Hooker, A.; Furdui, C.M.; Alli, E.; Li, W.; Donati, G.L.; et al. Silver nanoparticles selectively treat triple-negative breast cancer cells without affecting non-malignant breast epithelial cells in vitro and in vivo. FASEB Bioadv. 2019, 1, 639–660. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Salas, L.M.; Girón-Vázquez, N.G.; García-Ramos, J.C.; Torres-Bugarín, O.; Gómez, C.; Pestryakov, A.; Villarreal-Gómez, L.J.; Toledano-Magaña, Y.; Bogdanchikova, N. Antiproliferative and Antitumour Effect of Nongenotoxic Silver Nanoparticles on Melanoma Models. Oxid. Med. Cell. Longev. 2019, 2019, 4528241. [Google Scholar] [CrossRef] [PubMed]
- Tinajero-Díaz, E.; Salado-Leza, D.; Gonzalez, C.; Martínez Velázquez, M.; López, Z.; Bravo-Madrigal, J.; Knauth, P.; Flores-Hernández, F.Y.; Herrera-Rodríguez, S.E.; Navarro, R.E.; et al. Green Metallic Nanoparticles for Cancer Therapy: Evaluation Models and Cancer Applications. Pharmaceutics 2021, 13, 1719. [Google Scholar] [CrossRef] [PubMed]
- Alhadrami, H.A.; Alkhatabi, H.; Abduljabbar, F.H.; Abdelmohsen, U.R.; Sayed, A.M. Anticancer Potential of Green Synthesized Silver Nanoparticles of the Soft Coral Cladiella pachyclados Supported by Network Pharmacology and In Silico Analyses. Pharmaceutics 2021, 13, 1846. [Google Scholar] [CrossRef]
- Ahmed, O.; Sibuyi, N.R.S.; Fadaka, A.O.; Madiehe, M.A.; Maboza, E.; Meyer, M.; Geerts, G. Plant Extract-Synthesized Silver Nanoparticles for Application in Dental Therapy. Pharmaceutics 2022, 14, 380. [Google Scholar] [CrossRef]
- Khan, M.S.; Alomari, A.; Tabrez, S.; Hassan, I.; Wahab, R.; Bhat, S.A.; Alafaleq, N.O.; Altwaijry, N.; Shaik, G.M.; Zaidi, S.K.; et al. Anticancer Potential of Biogenic Silver Nanoparticles: A Mechanistic Study. Pharmaceutics 2021, 13, 707. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K. Aqueous Phase Lavender Leaf Mediated Green Synthesis of Gold Nanoparticles and Evaluation of its Antioxidant Activity. Biol. Med. 2016, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Bunghez, I.R.; Fierascu, R.C.; Dumitrescu, O.; Fierascu, I.; Ion, R.M. Characterization of silver nanoparticles obtained by Lavandula ANGUSTIFOLIA extract. Rev. Roum. Chim. 2015, 60, 515–519. [Google Scholar]
- Shipunova, V.O.; Deyev, S.M. Artificial Scaffold Polypeptides As an Efficient Tool for the Targeted Delivery of Nanostructures In Vitro and In Vivo. Acta Nat. 2022, 14, 31–49. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Sogomonyan, A.S.; Zelepukin, I.V.; Nikitin, M.P.; Deyev, S.M. PLGA Nanoparticles Decorated with Anti-HER2 Affibody for Targeted Delivery and Photoinduced Cell Death. Molecules 2021, 26, 3955. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Kolesnikova, O.A.; Kotelnikova, P.A.; Soloviev, V.D.; Popov, A.A.; Proshkina, G.M.; Nikitin, M.P.; Deyev, S.M. Comparative Evaluation of Engineered Polypeptide Scaffolds in HER2-Targeting Magnetic Nanocarrier Delivery. ACS Omega 2021, 6, 16000–16008. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Ukrainskaya, V.; Rubtsov, Y.; Pershin, D.; Podoplelova, N.; Terekhov, S.; Yaroshevich, I.; Sokolova, A.; Bagrov, D.; Kulakovskaya, E.; Shipunova, V.; et al. Antigen-Specific Stimulation and Expansion of CAR-T Cells Using Membrane Vesicles as Target Cell Surrogates. Small 2021, 17, e2102643. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, M.P.; Zelepukin, I.V.; Shipunova, V.O.; Sokolov, I.L.; Deyev, S.M.; Nikitin, P.I. Enhancement of the blood-circulation time and performance of nanomedicines via the forced clearance of erythrocytes. Nat. Biomed. Eng. 2020, 4, 717–731. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Shipunova, V.O.; Babenyshev, A.V.; Balalaeva, I.V.; Nikitin, P.I.; Deyev, S.M.; Nikitin, M.P. Nanoparticle-based drug delivery via RBC-hitchhiking for the inhibition of lung metastases growth. Nanoscale 2019, 11, 1636–1646. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shipunova, V.O.; Belova, M.M.; Kotelnikova, P.A.; Shilova, O.N.; Mirkasymov, A.B.; Danilova, N.V.; Komedchikova, E.N.; Popovtzer, R.; Deyev, S.M.; Nikitin, M.P. Photothermal Therapy with HER2-Targeted Silver Nanoparticles Leading to Cancer Remission. Pharmaceutics 2022, 14, 1013. https://doi.org/10.3390/pharmaceutics14051013
Shipunova VO, Belova MM, Kotelnikova PA, Shilova ON, Mirkasymov AB, Danilova NV, Komedchikova EN, Popovtzer R, Deyev SM, Nikitin MP. Photothermal Therapy with HER2-Targeted Silver Nanoparticles Leading to Cancer Remission. Pharmaceutics. 2022; 14(5):1013. https://doi.org/10.3390/pharmaceutics14051013
Chicago/Turabian StyleShipunova, Victoria O., Mariia M. Belova, Polina A. Kotelnikova, Olga N. Shilova, Aziz B. Mirkasymov, Natalia V. Danilova, Elena N. Komedchikova, Rachela Popovtzer, Sergey M. Deyev, and Maxim P. Nikitin. 2022. "Photothermal Therapy with HER2-Targeted Silver Nanoparticles Leading to Cancer Remission" Pharmaceutics 14, no. 5: 1013. https://doi.org/10.3390/pharmaceutics14051013