Resveratrol Microencapsulation into Electrosprayed Polymeric Carriers for the Treatment of Chronic, Non-Healing Wounds
Abstract
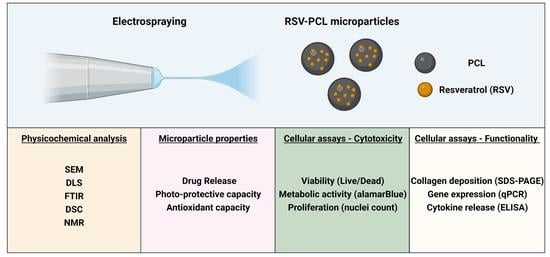
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Electrosprayed Particles
2.3. Morphological Analysis
2.4. Dynamic Light Scattering (DLS) Analysis
2.5. Fourier-Transform Infrared Spectroscopy (FT-IR) Spectroscopy Analysis
2.6. Differential Scanning Calorimetry (DSC) and Thermogravimetric (TGA) Analyses
2.7. Nuclear Magnetic Resonance (NMR) Spectroscopy Analysis
2.8. Resveratrol Loading Capacity and Encapsulation Efficiency Analyses
2.9. Photodegradation Analysis
2.10. Antioxidant Capacity Analysis
2.11. In Vitro Release Profile Analysis
2.12. Cell Culture
2.13. Cell Viability Analysis
2.14. Cell Proliferation Analysis
2.15. Cell Metabolic Activity Analysis
2.16. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
2.17. Immunocytochemistry Analysis
2.18. RNA Isolation and Gene Expression Analysis (RT-qPCR)
2.19. Multiplex Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
2.20. Statistical Analysis
3. Results
3.1. Morphological and Physicochemical Analyses of Electrosprayed Microparticles
3.2. Drug Content, Drug Release, Photodegradation and Antioxidant Analyses
3.3. Cell Attachment, Viability, Metabolic Activity, and Proliferation Analyses
3.4. Collagen Type I Deposition Analysis
3.5. Gene Expression, Multiplex ELISA and p65 Localization Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, C.K. Human wound and its burden: Updated 2020 compendium of estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Krishnaswamy, V.R.; Mintz, D.; Sagi, I. Matrix metalloproteinases: The sculptors of chronic cutaneous wounds. Biochim. Biophys. Acta 2017, 1864, 2220–2227. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: A systematic review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.S.; Smith, T.J.; Blieden, T.M.; Phipps, R.P. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of in-flammation. Am. J. Pathol. 1997, 151, 317–322. [Google Scholar]
- Buckley, C.D.; Pilling, D.; Lord, J.; Akbar, A.N.; Scheel-Toellner, D.; Salmon, M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001, 22, 199–204. [Google Scholar] [CrossRef]
- Filer, A.; Pitzalis, C.; Buckley, C.D. Targeting the stromal microenvironment in chronic inflammation. Curr. Opin. Pharmacol. 2006, 6, 393–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.; Kotzbeck, P. The impact of resveratrol on skin wound healing, scarring, and aging. Int. Wound J. 2022, 19, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Pyo, I.S.; Yun, S.; Yoon, Y.E.; Choi, J.-W.; Lee, S.-J. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Kaleci, B.; Koyuturk, M. Efficacy of resveratrol in the wound healing process by reducing oxidative stress and promoting fibroblast cell proliferation and migration. Dermatol. Ther. 2020, 33, e14357. [Google Scholar] [CrossRef]
- Frischholz, S.; Berberich, O.; Böck, T.; Meffert, R.H.; Blunk, T. Resveratrol counteracts IL-1β-mediated impairment of extracellular matrix deposition in 3D articular chondrocyte constructs. J. Tissue Eng. Regen. Med. 2020, 14, 897–908. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Zhao, P.; Sui, B.-D.; Liu, N.; Hu, C.-H.; Chen, J.; Zheng, C.-X.; Liu, A.-Q.; Xuan, K.; Pan, Y.-P.; et al. Resveratrol enhances the functionality and improves the regeneration of mesenchymal stem cell aggregates. Exp. Mol. Med. 2018, 50, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Gweon, E.J.; Kim, S.J. Resveratrol attenuates matrix metalloproteinase-9 and -2-regulated differentiation of HTB94 chon-drosarcoma cells through the p38 kinase and JNK pathways. Oncol. Rep. 2014, 32, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.-Y.; Hu, Y.; Guo, T.; Wang, H.-F.; Zhang, X.-P.; He, W.-J.; Tan, H. Resveratrol as a novel agent for treatment of multiple myeloma with matrix metalloproteinase inhibitory activity. Acta Pharmacol. Sin. 2006, 27, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Trotta, V.; Lee, W.-H.; Loo, C.-Y.; Haghi, M.; Young, P.M.; Scalia, S.; Traini, D. In vitro biological activity of resveratrol using a novel inhalable resveratrol spray-dried formulation. Int. J. Pharm. 2015, 491, 190–197. [Google Scholar] [CrossRef]
- Pashazadeh, H.; Zannou, O.; Ghellam, M.; Koca, I.; Galanakis, C.M.; Aldawoud, T.M.S. Optimization and Encapsulation of Phenolic Compounds Extracted from Maize Waste by Freeze-Drying, Spray-Drying, and Microwave-Drying Using Maltodextrin. Foods 2021, 10, 1396. [Google Scholar] [CrossRef]
- Shi, A.; Wang, J.; Guo, R.; Feng, X.; Ge, Y.; Liu, H.; Agyei, D.; Wang, Q. Improving resveratrol bioavailability using water-in-oil-in-water (W/O/W) emulsion: Physicochemical stability, in vitro digestion resistivity and transport properties. J. Funct. Foods 2021, 87, 104717. [Google Scholar] [CrossRef]
- Steipel, R.T.; Gallovic, M.D.; Batty, C.J.; Bachelder, E.M.; Ainslie, K.M. Electrospray for generation of drug delivery and vaccine particles applied in vitro and in vivo. Mater. Sci. Eng. C 2019, 105, 110070. [Google Scholar] [CrossRef]
- Wang, J.; Jansen, J.A.; Yang, F. Electrospraying: Possibilities and challenges of engineering carriers for biomedical applications-A mini review. Front. Chem. 2019, 7, 258. [Google Scholar] [CrossRef] [Green Version]
- Pawar, A.; Thakkar, S.; Misra, M. A bird’s eye view of nanoparticles prepared by electrospraying: Advancements in drug delivery field. J. Control. Release 2018, 286, 179–200. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, J. Electrosprayed polymer particles: Effect of the solvent properties. J. Appl. Polym. Sci. 2009, 114, 430–437. [Google Scholar] [CrossRef]
- Yu, D.-G.; Zhou, F.; Parker, G.J.M.; Simaite, A.; Buzgo, M.; Williams, G.R. Chapter Seven—Innovations and Advances in Electrospraying Technology. In Biomedical Applications of Electrospinning and Electrospraying; Kasoju, N., Ye, H., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 207–228. [Google Scholar]
- Boda, S.K.; Li, X.; Xie, J. Electrospraying an enabling technology for pharmaceutical and biomedical applications: A review. J. Aerosol Sci. 2018, 125, 164–181. [Google Scholar] [CrossRef] [PubMed]
- De Pieri, A.; Rana, S.; Korntner, S.; Zeugolis, D.I. Seaweed polysaccharides as macromolecular crowding agents. Int. J. Biol. Macromol. 2020, 164, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Coentro, J.Q.; May, U.; Prince, S.; Zwaagstra, J.; Ritvos, O.; Järvinen, T.A.; Zeugolis, D.I. Adapting the Scar-in-a-Jar to Skin Fibrosis and Screening Traditional and Contemporary Anti-Fibrotic Therapies. Front. Bioeng. Biotechnol. 2021, 9, 756399. [Google Scholar] [CrossRef] [PubMed]
- De Pieri, A.; Rochev, Y.; Zeugolis, D.I. Scaffold-free cell-based tissue engineering therapies: Advances, shortfalls and forecast. NPJ Regen. Med. 2021, 6, 18. [Google Scholar] [CrossRef]
- Gaspar, D.; Fuller, K.P.; Zeugolis, D.I. Polydispersity and negative charge are key modulators of extracellular matrix deposition under macromolecular crowding conditions. Acta Biomater. 2019, 88, 197–210. [Google Scholar] [CrossRef]
- Tsiapalis, D.; Zeugolis, D.I. It is time to crowd your cell culture media—Physicochemical considerations with biological consequences. Biomaterials 2021, 275, 120943. [Google Scholar] [CrossRef]
- Raghunath, M.; Zeugolis, D.I. Transforming eukaryotic cell culture with macromolecular crowding. Trends Biochem. Sci. 2021, 46, 805–811. [Google Scholar] [CrossRef]
- Towska, K.; Sobczak, M.; Olędzka, E. Novel zinc-catalytic systems for ring-opening polymerization of ε-caprolactone. Molecules 2015, 20, 2816–2827. [Google Scholar]
- Anal, T.; Koçak, İ.; Hazer, B. Synthesis of comb-type amphiphilic graft copolymers derived from chlorinated poly(ε-caprolactone) via click reaction. Polym. Bull. 2017, 74, 977–995. [Google Scholar]
- Holländer, J.; Genina, N.; Jukarainen, H.; Khajeheian, M.; Rosling, A.; Mäkilä, E.; Sandler, N. Three-Dimensional Printed PCL-Based Implantable Prototypes of Medical Devices for Controlled Drug Delivery. J. Pharm. Sci. 2016, 105, 2665–2676. [Google Scholar] [CrossRef] [Green Version]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Nature-Derived and Synthetic Additives to poly(ε-Caprolactone) Nanofibrous Systems for Biomedicine; an Updated Overview. Front. Chem. 2021, 9, 809676. [Google Scholar] [CrossRef] [PubMed]
- Bock, N.; Woodruff, M.A.; Hutmacher, D.W.; Dargaville, T.R. Electrospraying, a reproducible method for production of poly-meric microspheres for biomedical applications. Polymers 2011, 3, 131–149. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.R.; Wei, X.Q.; Song, X.; Hao, L.Y.; Cai, X.X.; Zhang, Z.R.; Peng, Q.; Lin, Y.F. Independent effect of polymeric nanoparticle zeta potential/surface charge, on their cytotoxicity and affinity to cells. Cell Prolif. 2015, 48, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.P.; Hendley, M.A.; Isely, C.; Annamalai, P.; Peña, E.A.; Gower, R.M. Resveratrol Delivery from Porous Poly (lactide-co-glycolide) Scaffolds Promotes an Anti-Inflammatory Environment within Visceral Adipose Tissue. ACS Appl. Mater. Interfaces 2018, 10, 43363–43374. [Google Scholar] [CrossRef]
- Rutledge, K.E.; Cheng, Q.; Jabbarzadeh, E. Modulation of Inflammatory Response and Induction of Bone Formation Based on Combinatorial Effects of Resveratrol. J. Nanomed. Nanotechnol. 2016, 7, 350. [Google Scholar] [CrossRef] [Green Version]
- Jayan, H.; Leena, M.M.; Sundari, S.S.; Moses, J.; Anandharamakrishnan, C. Improvement of bioavailability for resveratrol through encapsulation in zein using electrospraying technique. J. Funct. Foods 2019, 57, 417–424. [Google Scholar] [CrossRef]
- Cinan, E.; Cesur, S.; Haskoylu, M.E.; Gunduz, O.; Oner, E.T. Resveratrol-Loaded Levan Nanoparticles Produced by Electrohydrodynamic Atomization Technique. Nanomaterials 2021, 11, 2582. [Google Scholar] [CrossRef]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and incidence of chronic wounds and related complications: A protocol for a systematic review. Syst. Rev. 2016, 5, 152. [Google Scholar] [CrossRef] [Green Version]
- Werdin, F.; Tennenhaus, M.; Schaller, H.-E.; Rennekampff, H.-O. Evidence-based Management Strategies for Treatment of Chronic Wounds. Eplasty 2009, 9, e19. [Google Scholar] [PubMed]
- Broussard, K.C.; Powers, J.G. Wound Dressings: Selecting the Most Appropriate Type. Am. J. Clin. Dermatol. 2013, 14, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Huang, J.; Si, T.; Xu, R.X. Coaxial electrospray of microparticles and nanoparticles for biomedical applications. Expert Rev. Med. Devices 2012, 9, 595–612. [Google Scholar] [CrossRef] [Green Version]
- Si, T.; Zhang, L.; Li, G.; Roberts, C.J.; Yin, X.; Xu, R. Experimental design and instability analysis of coaxial electrospray process for microencapsulation of drugs and imaging agents. J. Biomed. Opt. 2013, 18, 075003. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Liu, W.; Jiang, P.; Hong, T. Coaxial electrohydrodynamic atomization for the production of drug-loaded mi-cro/nanoparticles. Micromachines 2019, 10, 125. [Google Scholar] [CrossRef] [Green Version]
- Kosović, E.; Topiař, M.; Cuřínová, P.; Sajfrtová, M. Stability testing of resveratrol and viniferin obtained from Vitis vinifera L. by various extraction methods considering the industrial viewpoint. Sci. Rep. 2020, 10, 5564. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.G.; Monteiro, J.; Marques, R.R.; Silva, A.M.; Martínez, C.; Canle, M.; Faria, J.L. Photochemical and photocatalytic degradation of trans-resveratrol. Photochem. Photobiol. Sci. 2013, 12, 638–644. [Google Scholar] [CrossRef]
- Borra, M.T.; Smith, B.; Denu, J.M. Mechanism of Human SIRT1 Activation by Resveratrol. J. Biol. Chem. 2005, 280, 17187–17195. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Wu, X.; Yang, Y.; Zhao, Y.; Fang, M.; Xie, W.; Wang, H.; Xu, Y. SIRT1 deacetylates RFX5 and antagonizes repression of collagen type I (COL1A2) transcription in smooth muscle cells. Biochem. Biophys. Res. Commun. 2012, 428, 264–270. [Google Scholar] [CrossRef]
- Christovam, A.C.; Theodoro, V.; Mendonça, F.A.S.; Esquisatto, M.A.M.; Dos Santos, G.M.T.; Amaral, M.E.C.D. Activators of SIRT1 in wound repair: An animal model study. Arch. Dermatol. Res. 2019, 311, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Elliott, C.G.; Hamilton, D.W. Deconstructing fibrosis research: Do pro-fibrotic signals point the way for chronic dermal wound regeneration? J. Cell Commun. Signal. 2011, 5, 301–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DesJardins-Park, H.E.; Gurtner, G.C.; Wan, D.C.; Longaker, M.T. From chronic wounds to scarring: The growing health care burden of under- and over-healing wounds. Adv. Wound Care 2021. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interf. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, R.; Rockel, J.S.; Kapoor, M.; Hinz, B. The inflammatory speech of fibroblasts. Immunol. Rev. 2021, 302, 126–146. [Google Scholar] [CrossRef]
- Ariga, A.; Namekawa, J.; Matsumoto, N.; Inoue, J.; Umezawa, K. Inhibition of tumor necrosis factor-alpha -induced nuclear translocation and activation of NF-kappa B by dehydroxymethylepoxyquinomicin. J. Biol. Chem. 2002, 277, 24625–24630. [Google Scholar] [CrossRef] [Green Version]
- Moreno, R.; Sobotzik, J.M.; Schultz, C.; Schmitz, M.L. Specification of the NF-kappaB transcriptional response by p65 phos-phorylation and TNF-induced nuclear translocation of IKK epsilon. Nucleic Acids Res. 2010, 38, 6029–6044. [Google Scholar] [CrossRef] [Green Version]
- Fuseler, J.W.; Merrill, D.M.; Rogers, J.A.; Grisham, M.B.; Wolf, R.E. Analysis and quantitation of NF-kappaB nuclear translocation in tumor necrosis factor alpha (TNF-alpha) activated vascular endothelial cells. Microsc. Microanal. 2006, 12, 269–276. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, L.; Cui, J.; Huoc, Z.; Xue, J.; Cui, H.; Mao, Q.; Yang, R. Resveratrol inhibits NF-kB signaling through suppression of p65 and IkappaB kinase activities. Pharmazie 2013, 68, 689–694. [Google Scholar]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Pukstad, B.S.; Ryan, L.; Flo, T.H.; Stenvik, J.; Moseley, R.; Harding, K.; Thomas, D.W.; Espevik, T. Non-healing is associated with persistent stimulation of the innate immune response in chronic venous leg ulcers. J. Dermatol. Sci. 2010, 59, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Dasu, M.R.; Isseroff, R.R. Toll-Like Receptors in Wound Healing: Location, Accessibility, and Timing. J. Investig. Dermatol. 2012, 132, 1955–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Alzahrani, M.Z.; Bakheet, S.A.; Attia, S.M. Resveratrol improves neuroimmune dysregu-lation through the inhibition of neuronal toll-like receptors and COX-2 signaling in BTBR T(+) Itpr3(tf)/J mice. Neuromol. Med. 2018, 20, 133–146. [Google Scholar] [CrossRef]
- Azam, S.; Jakaria, M.; Kim, I.-S.; Kim, J.; Haque, M.E.; Choi, D.-K. Regulation of Toll-Like Receptor (TLR) Signaling Pathway by Polyphenols in the Treatment of Age-Linked Neurodegenerative Diseases: Focus on TLR4 Signaling. Front. Immunol. 2019, 10, 1000. [Google Scholar] [CrossRef]
- Pignet, A.-L.; Schellnegger, M.; Hecker, A.; Kohlhauser, M.; Kotzbeck, P.; Kamolz, L.-P. Resveratrol-Induced Signal Transduction in Wound Healing. Int. J. Mol. Sci. 2021, 22, 12614. [Google Scholar] [CrossRef]
- Bollmann, F.; Hartmut, K.; Henke, J.; Schrick, K.; Besche, V.; Bros, M.; Li, H.; Siuda, D.; Handler, N.; Bauer, F.; et al. Resveratrol post-transcriptionally regulates pro-inflammatory gene expression via regulation of KSRP RNA binding activity. Nucleic Acids Res. 2014, 42, 12555–12569. [Google Scholar] [CrossRef]
- Cao, L.; Chen, X.; Xiao, X.; Ma, Q.; Li, W. Resveratrol inhibits hyperglycemia-driven ROS-induced invasion and migration of pancreatic cancer cells via suppression of the ERK and p38 MAPK signaling pathways. Int. J. Oncol. 2016, 49, 735–743. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Pieri, A.; Ocorr, K.; Jerreld, K.; Lamoca, M.; Hitzl, W.; Wuertz-Kozak, K. Resveratrol Microencapsulation into Electrosprayed Polymeric Carriers for the Treatment of Chronic, Non-Healing Wounds. Pharmaceutics 2022, 14, 853. https://doi.org/10.3390/pharmaceutics14040853
De Pieri A, Ocorr K, Jerreld K, Lamoca M, Hitzl W, Wuertz-Kozak K. Resveratrol Microencapsulation into Electrosprayed Polymeric Carriers for the Treatment of Chronic, Non-Healing Wounds. Pharmaceutics. 2022; 14(4):853. https://doi.org/10.3390/pharmaceutics14040853
Chicago/Turabian StyleDe Pieri, Andrea, Keegan Ocorr, Kyle Jerreld, Mikkael Lamoca, Wolfgang Hitzl, and Karin Wuertz-Kozak. 2022. "Resveratrol Microencapsulation into Electrosprayed Polymeric Carriers for the Treatment of Chronic, Non-Healing Wounds" Pharmaceutics 14, no. 4: 853. https://doi.org/10.3390/pharmaceutics14040853