Transdermal Permeation Assays of Curcumin Aided by CAGE-IL: In Vivo Control of Psoriasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Test Animals
2.1.2. Chemicals
2.2. Experimental Procedures
2.2.1. Synthesis of Choline and Geranic Acid Ionic Liquid (CAGE-IL)
2.2.2. Preparation and Characterization of Biopolysaccharide Gels
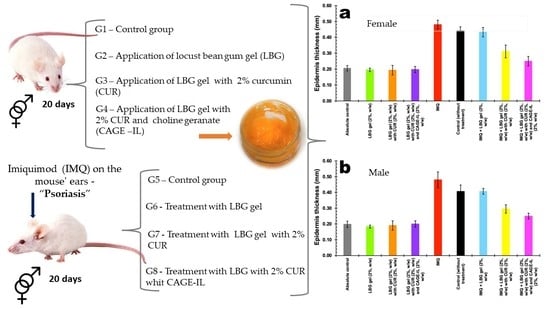
2.2.3. Experimental Design and Induction of Psoriasis
2.2.4. Assessment of Inflammation Severity
2.2.5. Mouse Euthanasia
2.2.6. Preparation of Histological Cuts
2.2.7. Statistical Analyses
3. Results
3.1. Thermal Characterization of Biopolysaccharide Gels via Differential Scanning Calorimetry (DSC) Analyses
3.2. Macroscopic/Microscopic Analysis of Mouse Ears
3.2.1. Control Groups
3.2.2. Induced Psoriasis Groups
3.3. Treatment of Psoriasis
3.4. Assessment of Mouse Ear Epidermis Thickness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.; Sarkar, M.K.; Tsoi, L.C.; Gudjonsson, J.E. Psoriasis: A mixed autoimmune and autoinflammatory disease. Curr. Opin. Immunol. 2017, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Calara, P.S.; Althin, R.; Carlsson, K.S.; Schmitt-Egenolf, M. Regional differences in the prescription of biologics for psoriasis in Sweden: A register-based Study of 4168 patients. BioDrugs 2017, 31, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniyal, M.; Akram, M.; Zainab, R.; Munir, N.; Shah, S.M.A.; Liu, B.; Wang, W.; Riaz, M.; Jabeen, F. Progress and prospects in the management of psoriasis and developments in phyto-therapeutic modalities. Dermatol. Ther. 2019, 32, e12866. [Google Scholar] [CrossRef] [PubMed]
- Dand, N.; Mahil, S.K.; Capon, F.; Smith, C.H.; Simpson, M.A.; Barker, J.N. Psoriasis and genetics. Acta Derm. Venereol. 2020, 100, 54–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; He, Y. The role of nociceptive neurons in the pathogenesis of psoriasis. Front. Immunol. 2020, 11, 1984. [Google Scholar] [CrossRef]
- Yamamoto, T. Psoriasis and connective tissue diseases. Int. J. Mol. Sci. 2020, 21, 5803. [Google Scholar] [CrossRef]
- Saleem, S.; Iqubal, M.K.; Garg, S.; Ali, J.; Baboota, S. Trends in nanotechnology-based delivery systems for dermal targeting of drugs: An enticing approach to offset psoriasis. Expert Opin. Drug Deliv. 2020, 17, 817–838. [Google Scholar] [CrossRef]
- Drvar, D.L.; Vlahinić, T.; Maleš, Ž.; Turčić, P.; Čeović, R. A modern approach to the treatment of plaque psoriasis. Acta Pharm. 2019, 69, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Gendrisch, F.; Haarhaus, B.; Krieger, N.; Quirin, K.-W.; Schempp, C.M.; Wölfle, U. The effect of herbal medicinal products on psoriasis-like keratinocytes. Biomolecules 2021, 11, 371. [Google Scholar] [CrossRef]
- Dutta, S.; Chawla, S.; Kumar, S. Psoriasis: A review of existing therapies and recent advances in treatment. J. Rational. Pharmacother. Res. 2018, 4, 12–23. [Google Scholar]
- Todke, P.; Shah, V.H. Psoriasis: Implication to disease and therapeutic strategies, with an emphasis on drug delivery approaches. Int. J. Dermatol. 2018, 57, 1387–1402. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Song, Z.; Wen, Y.; Xu, H.; Zhu, L.; Feng, R. Transdermal delivery of curcumin-loaded supramolecular hydrogels for dermatitis treatment. J. Mater. Sci. Mater. Med. 2019, 30, 11. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Cicero, A.F.G.; Panahi, Y.; Mohajeri, M.; Sahebkar, A. Curcumin: A naturally occurring autophagy modulator. J. Cell Physiol. 2019, 234, 5643–5654. [Google Scholar] [CrossRef]
- Nardo, V.D.; Gianfaldoni, S.; Tchernev, G.; Wollina, U.; Barygina, V.; Lotti, J.; Daaboul, F.; Lotti, T. Use of curcumin in psoriasis. Open Access Maced. J. Med. Sci. 2018, 6, 218–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raut, G.; Wairkar, S. Management of psoriasis with nutraceuticals: An update. Complement. Ther. Clin. Pract. 2018, 31, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Li, B.; Luo, L.; Jiang, W.; Lu, Q.; Rong, M.; Lai, R. Curcumin shows excellent therapeutic effect on psoriasis in mouse model. Biochimie 2016, 123, 73–80. [Google Scholar] [CrossRef]
- Todo, H. Transdermal permeation of drugs in various animal species. Pharmaceutics 2017, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, F.; Shafiee, M.; Banikazemi, Z.; Pourhanifeh, M.H.; Khanbabaei, H.; Shamshirian, A.; Moghadam, S.A.; Nezhad, R.A.; Sahebkar, A.; Avan, A.; et al. Curcumin inhibits NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathol. Res. Pract. 2019, 215, 152556. [Google Scholar] [CrossRef]
- Boscariol, R.; Caetano, É.A.; Silva, E.C.; Oliveira, T.J.; Rosa-Castro, R.M.; Vila, M.M.D.C.; Balcão, V.M. Performance of choline geranate deep eutectic solvent as transdermal permeation enhancer: An in vitro skin histological study. Pharmaceutics 2021, 13, 540. [Google Scholar] [CrossRef]
- Jorge, L.R.; Harada, L.K.; Silva, E.C.; Campos, W.F.; Moreli, F.C.; Shimamoto, G.; Pereira, J.F.B.; Oliveira, J.M., Jr.; Tubino, M.; Vila, M.M.D.C.; et al. Non-invasive transdermal delivery of human insulin using ionic liquids: In vitro studies. Front. Pharmacol. 2020, 11, 243. [Google Scholar] [CrossRef] [Green Version]
- Harada, L.K.; Pereira, J.F.B.; Campos, W.F.; Silva, E.C.; Moutinho, C.G.; Vila, M.M.D.C.; Oliveira, J.M., Jr.; Teixeira, J.A.; Balcão, V.M.; Tubino, M. Insights into protein-ionic liquid interactions aiming at macromolecule delivery systems. J. Braz. Chem. Soc. 2018, 29, 1983–1998. [Google Scholar] [CrossRef]
- Zakrewsky, M.; Lovejoy, K.S.; Kern, T.L.; Miller, T.E.; Le, V.; Nagy, A.; Goumas, A.M.; Iyer, R.S.; Del Sesto, R.E.; Koppisch, A.T.; et al. Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc. Natl. Acad. Sci. USA 2014, 111, 13313–13318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, A.; Ibsen, K.; Iwao, Y.; Zakrewsky, M.; Mitragotri, S. Transdermal protein delivery using choline and geranate (CAGE) deep eutectic solvent. Adv. Healthc. Mater. 2017, 6, 1601411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, W.F.; Silva, E.C.; Oliveira, T.J.; Oliveira, J.M., Jr.; Tubino, M.; Pereira, C.; Vila, M.M.D.C.; Balcão, V.M. Transdermal permeation of bacteriophage particles by choline oleate: Potential for treatment of soft-tissue infections. Future Microbiol. 2020, 15, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, M.; Boisgard, A.-S.; Danoy, A.; El Kholti, N.; Salvi, J.-P.; Boulieu, R.; Fromy, B.; Verrier, B.; Lamrayah, M. Advanced characterization of imiquimod-induced psoriasis-like mouse model. Pharmaceutics 2020, 12, 789. [Google Scholar] [CrossRef]
- Varma, S.R.; Sivaprakasam, T.O.; Mishra, A.; Prabhu, S.; Rafiq, M.; Rangesh, P. Imiquimod-induced psoriasis-like inflammation in differentiated human keratinocytes: Its evaluation using curcumin. Eur. J. Pharmacol. 2017, 813, 33–41. [Google Scholar] [CrossRef]
- Sawyer, L.M.; Malottki, K.; Sabry-Grant, C.; Yasmeen, N.; Wright, E.; Sohrt, A.; Borg, E.; Warren, R.B. Assessing the relative efficacy of interleukin- 17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: A systematic review and network meta-analysis of PASI response. PLoS ONE 2019, 14, e0220868. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xia, Q.; Li, Y.; He, Z.; Li, Z.; Guo, T.; Wu, Z.; Feng, N. CD44 assists the topical anti-psoriatic efficacy of curcumin-loaded hyaluronan-modified ethosomes: A new strategy for clustering drug in inflammatory skin. Theranostics 2019, 9, 48–64. [Google Scholar] [CrossRef]
- Li, Q.; Liu, W.; Gao, S.; Mao, Y.; Xin, Y. Application of imiquimod-induced murine psoriasis model in evaluating interleukin-17A antagonist. BMC Immunol. 2021, 22, 11. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Z.; Lin, Z.; Cun, D.; Tong, H.H.Y.; Yan, R.; Wang, R.; Zheng, Y. Comparison of normal versus imiquimod-induced psoriatic skin in mice for penetration of drugs and nanoparticles. Inter. J. Nanomed. 2018, 13, 5625–5635. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Zhang, X.; Xu, M.; Zhang, F.; Tian, F.; Cui, J.; Xia, Y.; Liang, C.; Zhou, S.; Wei, H.; et al. Berberine downregulates CDC6 and inhibits proliferation via targeting JAK-STAT3 signaling in keratinocytes. Cell Death Dis. 2019, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.-L.; Fan, Z.-L.; Yuan, J.-D.; Chen, P.-P.; Yang, J.-J.; Xu, J.; ZhuGe, D.-L.; Jin, B.-H.; Zhu, Q.-Y.; Shen, B.-X.; et al. Skin-penetrating polymeric nanoparticles incorporated in silk fibroin hydrogel for topical delivery of curcumin to improve its therapeutic effect on psoriasis mouse model. Colloids Surf. B Biointerfaces 2017, 160, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Boscariol, R.; Oliveira Junior, J.M.; Baldo, D.A.; Balcão, V.M.; Vila, M.M.D.C. Transdermal permeation of curcumin promoted by choline geranate ionic liquid: Potential for the treatment of skin diseases. Saudi. Pharm. J. 2022; in press. [Google Scholar] [CrossRef]
- Kang, N.-W.; Kim, M.-H.; Sohn, S.-Y.; Kim, K.-T.; Park, J.-H.; Lee, S.-Y.; Lee, J.-Y.; Kim, D.-D. Curcumin-loaded lipid-hybridized cellulose nanofiber film ameliorates imiquimod-induced psoriasis-like dermatitis in mice. Biomaterials 2018, 182, 245–258. [Google Scholar] [CrossRef]
- Islam, R.; Chowdhury, R.; Wakabayashi, R.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic liquid-in-oil microemulsions prepared with biocompatible choline carboxylic acids for improving the transdermal delivery of a sparingly soluble drug. Pharmaceutics 2020, 12, 392. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Baby, A.R.; Araújo, M.E.M.; Fernandes, A.S.; Costa, J.G.; Almeida, T.S. Choline-amino acid ionic liquids as green functional excipients to enhance drug solubility. Pharmaceutics 2018, 10, 288. [Google Scholar] [CrossRef] [Green Version]
- Yousef, S.A.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Benson, H.A.E.; Sakran, W.; Roberts, M.S. Mechanistic evaluation of enhanced curcumin delivery through human skin in vitro from optimised nanoemulsion formulations fabricated with different penetration enhancers. Pharmaceutics 2019, 11, 639. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.C.; Oliveira, T.J.; Moreli, F.C.; Harada, L.K.; Vila, M.M.D.C.; Balcão, V.M. Newly isolated lytic bacteriophages for Staphylococcus intermedius, structurally and functionally stabilized in a hydroxyethylcellulose gel containing choline geranate: Potential for transdermal permeation in veterinary phage therapy. Res. Vet. Sci. 2021, 135, 42–58. [Google Scholar] [CrossRef]
- Patel, N.A.; Patel, N.J.; Patel, R.P. Formulation and evaluation of curcumin gel for topical application. Pharm. Dev. Technol. 2008, 14, 83–92. [Google Scholar] [CrossRef]
- Colony Gums. Hydrocolloid and Stabilizer Systems. 2022. Available online: https://www.colonygums.com/uploads/COLONYGUMS_LOCUSTBEANGUM.pdf (accessed on 25 March 2022).
- Rocha, L.K.H.; Favaro, L.I.L.; Rios, A.C.; Silva, E.C.; Silva, W.F.; Stigliani, T.P.; Guilger, M.; Lima, R.; Oliveira, J.M., Jr.; Aranha, N.; et al. Sericin from Bombyx mori cocoons. Part I: Extraction and physicochemical-biological characterization for biopharmaceutical applications. Process Biochem. 2017, 61, 163–177. [Google Scholar] [CrossRef]
- Choi, M.R.; Kim, H.D.; Cho, S.; Jeon, S.H.; Kim, D.H.; Wee, J.; Yang, Y.D. Anoctamin1 induces hyperproliferation of HaCaT keratinocytes and triggers imiquimod-induced psoriasis-like skin injury in mice. Int. J. Mol. Sci. 2021, 22, 7145. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, Z.; Wang, L.; Cun, D.; Tong, H.H.Y.; Yan, R.; Chen, X.; Wang, R.; Zheng, Y. Enhanced topical penetration, system exposure and anti-psoriasis activity of two particle-sized, curcumin-loaded PLGA nanoparticles in hydrogel. J. Control. Release 2017, 254, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Matza, L.S.; Brazier, J.E.; Stewart, K.D.; Pinto, L.; Bender, R.H.; Kircik, L.; Jordan, J.; Kim, K.J.; Mutebi, A.; Viswanathan, H.N.; et al. Developing a preference-based utility scoring algorithm for the Psoriasis Area Severity Index (PASI). J. Med. Econ. 2019, 22, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Hesselvig, J.H.; Egeberg, A.; Loft, N.D.; Zachariae, C.; Kofoed, K.; Skov, L. Correlation between dermatology life quality index and psoriasis area and severity index in patients with psoriasis treated with ustekinumab. Acta Derm. Venereol. 2018, 98, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Filippone, A.; Consoli, G.M.L.; Granata, G.; Casili, G.; Lanza, M.; Ardizzone, A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Topical delivery of curcumin by choline-calix[4]arene-based nanohydrogel improves its therapeutic effect on a psoriasis mouse model. Int. J. Mol. Sci. 2020, 21, 5053. [Google Scholar] [CrossRef]
- Nguyen, L.T.H.; Ahn, S.-H.; Nguyen, U.T.; Yang, I.-J. Dang-Gui-Liu-Huang Tang a traditional herbal formula, ameliorates imiquimod-induced psoriasis-like skin inflammation in mice by inhibiting IL-22 production. Phytomedicine 2018, 47, 48–57. [Google Scholar] [CrossRef]
- Alturkistani, H.A.; Tashkandi, F.M.; Mohammedsaleh, Z.M. Histological stains: A literature review and case study. Glob. J. Health Sci. 2016, 8, 72–79. [Google Scholar] [CrossRef]
- de Haan, K.; Zhang, Y.; Zuckerman, J.E.; Liu, T.; Sisk, A.E.; Diaz, M.F.P.; Jen, K.-Y.; Nobori, A.; Liou, S.; Zhang, S.; et al. Deep learning-based transformation of H&E stained tissues into special stains. Nat. Commun. 2021, 12, 4884. [Google Scholar] [CrossRef]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- MudgiL, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zhang, Z.; McClements, D.J. Nanoemulsions: An emerging platform for increasing the efficacy of nutraceuticals in foods. Colloids Surf. B Biointerfaces 2020, 194, 111202. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, F.S.; Kadklodaee, R.; Emadzadeh, B.; Koocheki, A. Preparation and characterization of tragacanth-locust bean gum edible blend films. Carbohydr. Polym. 2016, 139, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Dionísio, M.; Grenha, A. Locust bean gum: Exploring its potential for biopharmaceutical applications. J. Pharm. Bioallied Sci. 2012, 4, 175–185. [Google Scholar] [PubMed]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential scanning calorimetry techniques: Applications in biology and nanoscience. J. Biomol. Tech. 2010, 21, 167–193. [Google Scholar] [PubMed]
- Schön, M.P.; Manzke, V.; Erpenbeck, L. Animal models of psoriasis—highlights and drawbacks. J. Allergy Clin. Immunol. 2021, 147, 439–455. [Google Scholar] [CrossRef]
- Swindell, W.R.; Michaels, K.A.; Sutter, A.J.; Diaconu, D.; Fritz, Y.; Xing, X.; Sarkar, M.K.; Liang, Y.; Tsoi, A.; Gudjonsson, J.E.; et al. Imiquimod has strain-dependent effects in mice and does not uniquely model human psoriasis. Genome Med. 2017, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Smarr, B.L.; Grant, A.D.; Zucker, I.; Prendergast, B.J.; Kriegsfeld, L.J. Sex differences in variability across timescales in BALB/c mice. Biol. Sex Differ. 2017, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Curreri, A.M.; Mitagotri, S.; Tanner, E.E.L. Recent advances in ionic liquids in biomedicine. Adv. Sci. 2021, 8, 2004819. [Google Scholar] [CrossRef]
- Alzeer, F.; Alotair, H. Epidemiology and Cutaneous Manifestations of Psoriasis in Saudi Arabia: A Narrative Review. Clin. Cosmet. Investig. Dermatol. 2022, 15, 347–355. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Ryan, C.; Murase, J.E. Clinical considerations for the management of psoriasis in women. Int. J. Women’s Dermatol. 2019, 5, 141–150. [Google Scholar] [CrossRef]
- Wu, S.; Cho, E.; Li, W.; Grodstein, F.; Qureshi, A.A. Hormonal factors and risk of psoriasis in women: A cohort study. Acta Derm. Venereol. 2016, 96, 927–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Compound | Function in the Formulation | Formulation | |||||
|---|---|---|---|---|---|---|---|
| {LBG} Gel | {LBG + CUR} Gel | {LBG + CUR + CAGE − IL} Gel | {LBG} Gel | {LBG + CUR} Gel | {LBG+ CUR + CAGE − IL} Gel | ||
| % (w/w) | Mass (g) | ||||||
| Locust bean gum (LBG) | Gel-forming agent | 2 | 2 | 2 | 2.000 | 2.000 | 2.000 |
| Curcumin (CUR) | Bioactive compound | ----- | 2 | 2 | 2.000 | 2.000 | 2.000 |
| Choline and geranic acid ionic liquid (CAGE-IL) | Skin permeation enhancer | ----- | ----- | 2 | ----- | ----- | 2.000 |
| Methylparaben | Preservative, antifungal | 0.1 | 0.1 | 0.1 | 0.100 | 0.100 | 0.100 |
| Ultrapure water | Solvent | up to 100 | up to 100 | up to 100 | up to 100 | up to 100 | up to 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boscariol, R.; Caetano, É.A.; Grotto, D.; Rosa-Castro, R.M.; Oliveira Junior, J.M.; Vila, M.M.D.C.; Balcão, V.M. Transdermal Permeation Assays of Curcumin Aided by CAGE-IL: In Vivo Control of Psoriasis. Pharmaceutics 2022, 14, 779. https://doi.org/10.3390/pharmaceutics14040779
Boscariol R, Caetano ÉA, Grotto D, Rosa-Castro RM, Oliveira Junior JM, Vila MMDC, Balcão VM. Transdermal Permeation Assays of Curcumin Aided by CAGE-IL: In Vivo Control of Psoriasis. Pharmaceutics. 2022; 14(4):779. https://doi.org/10.3390/pharmaceutics14040779
Chicago/Turabian StyleBoscariol, Rodrigo, Érika A. Caetano, Denise Grotto, Raquel M. Rosa-Castro, José M. Oliveira Junior, Marta M. D. C. Vila, and Victor M. Balcão. 2022. "Transdermal Permeation Assays of Curcumin Aided by CAGE-IL: In Vivo Control of Psoriasis" Pharmaceutics 14, no. 4: 779. https://doi.org/10.3390/pharmaceutics14040779