Escinosomes: Safe and Successful Nanovesicles to Deliver Andrographolide by a Subcutaneous Route in a Mice Model of Oxaliplatin-Induced Neuropathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPLC-DAD Analytical Method
2.3. Preparation of Escinosomes
2.4. Physical Characterization of Escinosomes
2.4.1. Dynamic and Electrophoretic Light Scattering
2.4.2. Cryo Transmission Electron Microscopy
2.5. Chemical Characterization of Escinosomes
2.6. Release Study of ESN and AG from Escinosomes
2.6.1. Selection of Acceptor Medium
2.6.2. Drug Release Experiment
2.7. Stability Studies
2.8. Animals
2.9. Oxaliplatin-Induced Neuropathic Pain Model and Andrographolide Administration
2.10. Assessment of Thermal Allodynia (Cold Plate Test)
2.11. Irwin Test
2.12. Statistical Analysis
3. Results
3.1. Preparation and Light Scattering Analysis of Escinosomes
3.2. Physical Characterization of Escinosomes by Cryo Transmission Electron Microscopy
3.3. Chemical Characterization of Escinosomes
3.4. Stability Study of Escinosomes
3.5. Selection of the Acceptor Medium and Release Study
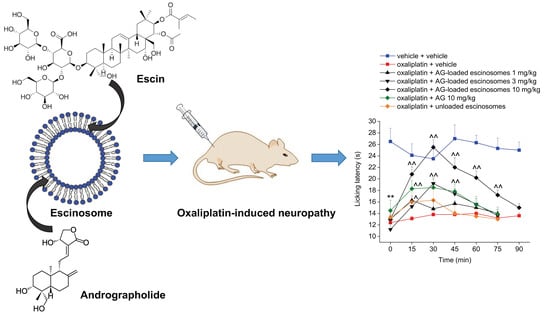
3.6. In Vivo Evaluation of AG-Loaded Escinosomes Efficacy against Neuropathic Pain
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; Macleod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Hausheer, F.H.; Schilsky, R.L.; Bain, S.; Berghorn, E.J.; Lieberman, F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin. Oncol. 2006, 33, 15–49. [Google Scholar] [CrossRef]
- Cruccu, G.; Truini, A. A review of neuropathic pain: From guidelines to clinical practice. Pain Ther. 2017, 6, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Nardiello, P.; Piazzini, V.; Leri, M.; Bergonzi, M.C.; Bucciantini, M.; Casamenti, F. Successful brain delivery of andrographolide loaded in human albumin nanoparticles to TgCRND8 mice, an Alzheimer’s disease mouse model. Front. Pharmacol. 2019, 10, 910. [Google Scholar] [CrossRef] [PubMed]
- Asprea, M.; Tatini, F.; Piazzini, V.; Rossi, F.; Bergonzi, M.C.; Bilia, A.R. Stable, monodisperse, and highly cell-permeating nanocochleates from natural soy lecithin liposomes. Pharmaceutics 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Chellampillai, B.; Pawar, A.P. Improved bioavailability of orally administered andrographolide from pH-sensitive nanoparticles. Eur. J. Drug Metab. Pharmacokinet. 2011, 35, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Guccione, C.; Oufir, M.; Piazzini, V.; Eigenmann, D.E.; Jähne, E.A.; Zabela, V.; Faleschini, M.T.; Bergonzi, M.C.; Smiesko, M.; Hamburger, M.; et al. Andrographolide-loaded nanoparticles for brain delivery: Formulation, characterisation and in vitro permeability using HCMEC/D3 cell line. Eur. J. Pharm. Biopharm. 2017, 119, 253–263. [Google Scholar] [CrossRef]
- Casamonti, M.; Risaliti, L.; Vanti, G.; Piazzini, V.; Bergonzi, M.C.; Bilia, A.R. Andrographolide loaded in micro- and nano-formulations: Improved bioavailability, target-tissue distribution, and efficacy of the “king of bitters”. Engineering 2019, 5, 569–575. [Google Scholar] [CrossRef]
- Vanti, G. Recent strategies in nanodelivery systems for natural products: A review. Environ. Chem. Lett. 2021, 19, 4311–4326. [Google Scholar] [CrossRef]
- Vanti, G.; Bani, D.; Salvatici, M.C.; Bergonzi, M.C.; Bilia, A.R. Development and percutaneous permeation study of escinosomes, escin-based nanovesicles loaded with berberine chloride. Pharmaceutics 2019, 11, 682. [Google Scholar] [CrossRef]
- Vanti, G.; Mannelli, L.D.C.; Micheli, L.; Cinci, L.; Grifoni, L.; Bergonzi, M.C.; Ghelardini, C.; Bilia, A.R. The anti-arthritic efficacy of khellin loaded in ascorbyl decanoate nanovesicles after an intra-articular administration. Pharmaceutics 2021, 13, 1275. [Google Scholar] [CrossRef] [PubMed]
- Vanti, G.; Grifoni, L.; Bergonzi, M.C.; Antiga, E.; Montefusco, F.; Caproni, M.; Bilia, A.R. Development and optimisation of biopharmaceutical properties of a new microemulgel of cannabidiol for locally-acting dermatological delivery. Int. J. Pharm. 2021, 607, 121036. [Google Scholar] [CrossRef] [PubMed]
- Vanti, G.; Muti, L.; D’Ambrosio, M.; Grifoni, L.; Bergonzi, M.C.; Luceri, C.; Bilia, A.R. Nanostructured lipid carriers can enhance oral absorption of khellin, a natural pleiotropic molecule. Molecules 2021, 26, 7657. [Google Scholar] [CrossRef] [PubMed]
- Vanti, G.; Tomou, E.-M.; Stojković, D.; Ćirić, A.; Bilia, A.R.; Skaltsa, H. Nanovesicles loaded with origanum onites and satureja thymbra essential oils and their activity against food-borne pathogens and spoilage microorganisms. Molecules 2021, 26, 2124. [Google Scholar] [CrossRef]
- Vanti, G.; Coronnello, M.; Bani, D.; Mannini, A.; Bergonzi, M.C.; Bilia, A.R. Co-delivery of berberine chloride and tariquidar in nanoliposomes enhanced intracellular berberine chloride in a doxorubicin-resistant K562 cell line due to P-Gp overexpression. Pharmaceutics 2021, 13, 306. [Google Scholar] [CrossRef]
- Vanti, G.; Ntallis, S.G.; Panagiotidis, C.A.; Dourdouni, V.; Patsoura, C.; Bergonzi, M.C.; Lazari, D.; Bilia, A.R. Glycerosome of Melissa officinalis L. essential oil for effective anti-HSV type 1. Molecules 2020, 25, 3111. [Google Scholar] [CrossRef]
- Micheli, L.; di Cesare Mannelli, L.; Rizzi, A.; Guerrini, R.; Trapella, C.; Calò, G.; Ghelardini, C. Intrathecal administration of Nociceptin/Orphanin FQ receptor agonists in rats: A strategy to relieve chemotherapy-induced neuropathic hypersensitivity. Eur. J. Pharmacol. 2015, 766, 155–162. [Google Scholar] [CrossRef]
- Lucarini, E.; Micheli, L.; Trallori, E.; Citi, V.; Martelli, A.; Testai, L.; de Nicola, G.R.; Iori, R.; Calderone, V.; Ghelardini, C.; et al. Effect of glucoraphanin and sulforaphane against chemotherapy-induced neuropathic pain: Kv7 potassium channels modulation by H2S release in vivo. Phytother. Res. 2018, 32, 2226–2234. [Google Scholar] [CrossRef]
- Falsini, M.; Catarzi, D.; Varano, F.; Ceni, C.; Dal Ben, D.; Marucci, G.; Buccioni, M.; Volpini, R.; di Cesare Mannelli, L.; Lucarini, E.; et al. Antioxidant-conjugated 1,2,4-triazolo[4,3-a]pyrazin-3-one derivatives: Highly potent and selective human A2A adenosine receptor antagonists possessing protective efficacy in neuropathic pain. J. Med. Chem. 2019, 62, 8511–8531. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Oussoren, C.; Storm, G. Liposomes to target the lymphatics by subcutaneous administration. Adv. Drug Deliv. Rev. 2001, 50, 143–156. [Google Scholar] [CrossRef]
- Badiee, A.; Khamesipour, A.; Samiei, A.; Soroush, D.; Shargh, V.H.; Kheiri, M.T.; Barkhordari, F.; Mc Master, W.R.; Mahboudi, F.; Jaafari, M.R. The role of liposome size on the type of immune response induced in BALB/c mice against leishmaniasis: Rgp63 as a model antigen. Exp. Parasitol. 2012, 132, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Burgos, R.A.; Hancke, J.L.; Bertoglio, J.C.; Aguirre, V.; Arriagada, S.; Calvo, M.; Cáceres, D.D. Efficacy of an andrographis paniculata composition for the relief of rheumatoid arthritis symptoms: A prospective randomized placebo-controlled trial. Clin. Rheumatol. 2009, 28, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.R.; Zakaria, Z.A.; Rahman, A.A.; Mohamad, A.S.; Desa, M.N.; Stanslas, J.; Moin, S.; Israf, D.A. Antinociceptive and antiedematogenic activities of andrographolide isolated from Andrographis paniculata in animal models. Biol. Res. Nurs. 2010, 11, 293–301. [Google Scholar] [CrossRef]
- Gupta, S.; Mishra, K.P.; Singh, S.B.; Ganju, L. Inhibitory effects of andrographolide on activated macrophages and adjuvant-induced arthritis. Inflammopharmacology 2018, 26, 447–456. [Google Scholar] [CrossRef]
- Wang, R.; Li, J.; Xu, X.; Xu, J.; Jiang, H.; Lv, Z.; Wu, R.; Sun, Z.; Guo, W.; Sun, Y.; et al. Andrographolide attenuates synovial inflammation of osteoarthritis by interacting with tumor necrosis factor receptor 2 trafficking in a rat model. J. Orthop. Transl. 2021, 29, 89–99. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, X.; Guo, S.W. Therapeutic potential of andrographolide for treating endometriosis. Hum. Reprod. 2012, 27, 1300–1313. [Google Scholar] [CrossRef]
- Gao, J.; Cui, J.; Zhong, H.; Li, Y.; Liu, W.; Jiao, C.; Gao, J.; Jiang, C.; Guo, W.; Xu, Q. Andrographolide sulfonate ameliorates chronic colitis induced by TNBS in mice via decreasing inflammation and fibrosis. Int. Immunopharmacol. 2020, 83, 106426. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, N.; Wang, Y.; Wang, Y.; Wu, C.; Cheng, X.; Wang, C. Improvement of oxazolone-induced ulcerative colitis in rats using andrographolide. Molecules 2020, 25, 76. [Google Scholar] [CrossRef]
- Yi, Z.; Ouyang, S.; Zhou, C.; Xie, L.; Fang, Z.; Yuan, H.; Yang, J.; Zou, L.; Jia, T.; Zhao, S.; et al. Andrographolide inhibits mechanical and thermal hyperalgesia in a rat model of HIV-induced neuropathic pain. Front. Pharmacol. 2018, 9, 593. [Google Scholar] [CrossRef]
- Wang, H.C.; Tsay, H.S.; Shih, H.N.; Chen, Y.A.; Chang, K.M.; Agrawal, D.C.; Huang, S.; Lin, Y.L.; Lee, M.J. Andrographolide relieved pathological pain generated by spared nerve injury model in mice. Pharm. Biol. 2018, 56, 124–131. [Google Scholar] [CrossRef]
- Panossian, A.; Hovhannisyan, A.; Mamikonyan, G.; Abrahamian, H.; Hambardzumyan, E.; Gabrielian, E.; Goukasova, G.; Wikman, G.; Wagner, H. Pharmacokinetic and oral bioavailability of andrographolide from andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine 2000, 7, 351–364. [Google Scholar] [CrossRef]
- Yunos, N.M.; Beale, P.; Yu, J.Q.; Huq, F. Synergism from the combination of oxaliplatin with selected phytochemicals in human ovarian cancer cell lines. Anticancer. Res. 2011, 31, 4283–4289. [Google Scholar] [PubMed]
- Su, M.; Qin, B.; Liu, F.; Chen, Y.; Zhang, R. Andrographolide enhanced 5-fluorouracil-induced antitumor effect in colorectal cancer via inhibition of c-MET pathway. Drug Des. Dev. Ther. 2017, 11, 3333–3341. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE Etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef]






| Formulation | Hydration Volume (mL) | Sonication Time and Amplitude (min -%) | Equilibration Time at 25 °C (min) | Temperature of 1-Night Storage (°C) | Size (nm) | PdI |
|---|---|---|---|---|---|---|
| 1 | 5 | 0 | 0 | NS | 342.4 ± 11.80 | 0.377 ± 0.0531 |
| 2 | 5 | 5–48 | 30 | NS | 142.4 ± 0.1381 | 0.426 ± 0.0217 |
| 3 | 5 | 5–48 | 60 | NS | 135.1 ± 0.6249 | 0.416 ± 0.00320 |
| 4 | 5 | 5–48 | 60 | 4 | 112.2 ± 2.185 | 0.362 ± 0.0110 |
| 5 | 5 | 5–48 | 60 | 25 | 136.5 ± 3.510 | 0.363 ± 0.0192 |
| 6 | 5 | 10–48 | 30 | NS | 101.4 ± 12.72 | 0.500 ± 0.0586 |
| 7 | 5 | 10–48 | 60 | NS | 120.9 ± 13.91 | 0.608 ± 0.0651 |
| 8 | 5 | 10–48 | 60 | 4 | 122.9 ± 13.72 | 0.526 ± 0.116 |
| 9 | 5 | 10–48 | 60 | 25 | 142.4 ± 11.32 | 0.589 ± 0.0720 |
| 10 | 10 | 0 | 0 | NS | 761.4 ± 39.81 | 0.545 ± 0.0913 |
| 11 | 10 | 1–30 | 15 | NS | 259.3 ± 3.574 | 0.451 ± 0.0187 |
| 12 | 10 | 5–30 | 15 | NS | 111.3 ± 3.156 | 0.421 ± 0.0123 |
| 13 | 10 | 10–30 | 15 | NS | 88.10 ± 0.921 | 0.387 ± 0.0130 |
| 14 | 10 | 10–48 | 15 | 4 | 164.7 ± 13.31 | 0.190 ± 0.0891 |
| R% | EE% | |
|---|---|---|
| ESN | 98.4 ± 4.63 | 96.7 ± 1.98 |
| AG | 97.0 ± 2.81 | 88.3 ± 1.43 |
| Acceptor Medium | AG Solubility (mg/mL) | S/C Ratio |
|---|---|---|
| PBS | 0.0582 ± 0.000805 | 3 |
| 1% v/v Tween 20/PBS | 0.0912 ± 0.00967 | 5 |
| 5% v/v Tween 20/PBS | 0.213 ± 0.000782 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanti, G.; Capizzi, M.; Di Cesare Mannelli, L.; Lucarini, E.; Bergonzi, M.C.; Ghelardini, C.; Bilia, A.R. Escinosomes: Safe and Successful Nanovesicles to Deliver Andrographolide by a Subcutaneous Route in a Mice Model of Oxaliplatin-Induced Neuropathy. Pharmaceutics 2022, 14, 493. https://doi.org/10.3390/pharmaceutics14030493
Vanti G, Capizzi M, Di Cesare Mannelli L, Lucarini E, Bergonzi MC, Ghelardini C, Bilia AR. Escinosomes: Safe and Successful Nanovesicles to Deliver Andrographolide by a Subcutaneous Route in a Mice Model of Oxaliplatin-Induced Neuropathy. Pharmaceutics. 2022; 14(3):493. https://doi.org/10.3390/pharmaceutics14030493
Chicago/Turabian StyleVanti, Giulia, Michela Capizzi, Lorenzo Di Cesare Mannelli, Elena Lucarini, Maria Camilla Bergonzi, Carla Ghelardini, and Anna Rita Bilia. 2022. "Escinosomes: Safe and Successful Nanovesicles to Deliver Andrographolide by a Subcutaneous Route in a Mice Model of Oxaliplatin-Induced Neuropathy" Pharmaceutics 14, no. 3: 493. https://doi.org/10.3390/pharmaceutics14030493