Nanocarriers Based on Gold Nanoparticles for Epigallocatechin Gallate Delivery in Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
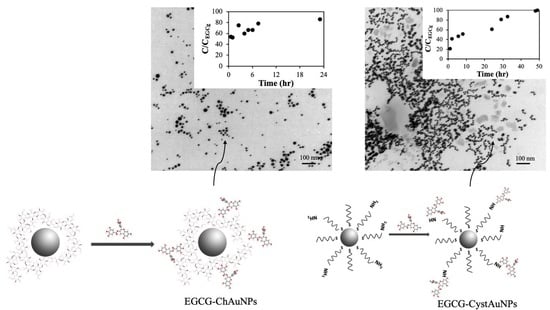
2.2. Conjugation of EGCG with Gold Nanoparticles Synthetized with Chitosan (EGCG-ChAuNPs)
2.3. Conjugation of EGCG with CystAuNPs (EGCG-CystAuNPs)
2.4. Dynamic Light Scattering
2.5. Transmission Electron Microscopy (TEM)
2.6. UV-Vis Spectroscopy
2.7. EGCG Encapsulation Efficiency
2.8. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.9. In Vitro Release Studies
2.10. Antioxidant Assay
2.11. Cell Culture
2.12. In Vitro Cytotoxicity Evaluation
2.13. Caspase-3 Activation Assay
2.14. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, Q.; Zhang, J.; Gao, J.; Zhang, Z.; Zhu, H.; Wang, D. Gold Nanoparticles in Cancer Theranostics. Front. Bioeng. Biotechnol. 2021, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.C.; Reis, D.P.; Pereira, M.C.; Coelho, M.A.N. Gold Nanoparticles for Targeting Varlitinib to Human Pancreatic Cancer Cells. Pharmaceutics 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Lu, G. Aplications of Gold Nanoparticles in Cancer Imaging and Treatment. In Applications of Gold Nanoparticles in Cancer Imaging and Treatment, Noble and Precious Metals—Properties, Nanoscale Effects and Applications; Seehra, M.S., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Al Shabrmi, F.M.; Allemailem, K.S.; Aly, S.M.; Khan, M.A. Implications of Green Tea and Its Constituents in the Prevention of Cancer via the Modulation of Cell Signalling Pathway. BioMed Res. Int. 2015, 2015, 925640. [Google Scholar] [CrossRef] [PubMed]
- Safwat, M.A.; Kandil, B.A.; Elblbesy, M.A.; Soliman, G.M.; Eleraky, N.E. Epigallocatechin-3-Gallate-Loaded Gold Nanoparticles: Preparation and Evaluation of Anticancer Efficacy in Ehrlich Tumor-Bearing Mice. Pharmaceuticals 2020, 13, 254. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Nallappan, D.; Madhavi, K.; Rahman, S.; Jun Wei, L.; Gan, S.H. Phytochemicals and Biogenic Metallic Nanoparticles as Anticancer Agents. Oxid. Med. Cell. Longev. 2016, 2016, 3685671. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Teng, C.; Min, Q. Advanced Nanovehicles-Enabled Delivery Systems of Epigallocatechin Gallate for Cancer Therapy. Front. Chem. 2020, 8, 874. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, M.-J.; Cha, S.-H.; Kim, Y.S.; Cho, S.; Park, Y. Catechin-capped gold nanoparticles: Green synthesis, characterization, and catalytic activity toward 4-nitrophenol reduction. Nanoscale Res. Lett. 2014, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Sanna, V.; Pala, N.; Dessì, G.; Manconi, P.; Mariani, A.; Dedola, S.; Rassu, M.; Crosio, C.; Iaccarino, C.; Sechi, M. Single-step green synthesis and characterization of gold-conjugated polyphenol nanoparticles with antioxidant and biological activities. Int. J. Nanomed. 2014, 9, 4935–4951. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, S.M.; Gamal-Eldeen, A.M.; Maksoud, N.A.E.; Fahmi, A.A. Epigallocatechin gallate-capped gold nanoparticles enhanced the tumor suppressors let-7a and miR-34a in hepatocellular carcinoma cells. An. Acad. Bras. Ciências 2020, 92, e20200574. [Google Scholar] [CrossRef]
- Rocha, S.; Generalov, R.; Pereira, M.D.C.; Peres, I.; Juzenas, P.; Coelho, M.A.N. Epigallocatechin gallate-loaded polysaccharide nanoparticles for prostate cancer chemoprevention. Nanomedicine 2010, 6, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Huang, J.; Feng, Q.; Chen, X.; Liu, X.; Li, X.; Zhang, T.; Xiao, S.; Li, H.; Zhong, Z.; et al. Size- and cell type-dependent cellular uptake, cytotoxicity and in vivo distribution of gold nanoparticles. Int. J. Nanomed. 2019, 14, 6957–6970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.C.; Almeida, G.M.; Pereira, M.C.; Santos-Silva, F.; Coelho, M.A.N. Functionalized gold nanoparticles improve afatinib delivery into cancer cells. Expert Opin. Drug Deliv. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Boyles, M.S.P.; Kristl, T.; Andosch, A.; Zimmermann, M.; Tran, N.; Casals, E.; Himly, M.; Puntes, V.; Huber, C.G.; Lütz-Meindl, U.; et al. Chitosan functionalisation of gold nanoparticles encourages particle uptake and induces cytotoxicity and pro-inflammatory conditions in phagocytic cells, as well as enhancing particle interactions with serum components. J. Nanobiotechnol. 2015, 13, 84. [Google Scholar] [CrossRef] [Green Version]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef]
- Fujisawa, T.; Rubin, B.; Suzuki, A.; Patel, P.S.; Gahl, W.A.; Joshi, B.H.; Puri, R.K. Cysteamine suppresses invasion, metastasis and prolongs survival by inhibiting matrix metalloproteinases in a mouse model of human pancreatic cancer. PLoS ONE 2012, 7, e34437. [Google Scholar] [CrossRef]
- Rubin, B.; Gilbert, M.; Jung, J. Use of Cysteamine and Derivatives Thereof to Supress Tumor Metastases. U.S. Patent 20210290569, 2 June 2021. [Google Scholar]
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin Loaded on Gold Nanoparticles as a Drug Delivery System for a Successful Biocompatible, Anti-Cancer, Anti-Inflammatory and Phagocytosis Inducer Model. Sci. Rep. 2020, 10, 9362. [Google Scholar] [CrossRef]
- Leu, J.-G.; Chen, S.-A.; Chen, H.-M.; Wu, W.-M.; Hung, C.-F.; Yao, Y.-D.; Tu, C.-S.; Liang, Y.-J. The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and α-lipoic acid. Nanomedicine 2011, 8, 767–775. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, Z.; Liu, R.; Liu, L.; Li, H.; Li, X.; Chen, Y.; Lu, C.; Liu, Y. AuNPs as an important inorganic nanoparticle applied in drug carrier systems. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4222–4233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattarai, S.R.; Kc, R.B.; Aryal, S.; Bhattarai, N.; Kim, S.Y.; Yi, H.K.; Hwang, P.H.; Kim, H.Y. Hydrophobically modified chitosan/gold nanoparticles for DNA delivery. J. Nanoparticle Res. 2008, 10, 151–162. [Google Scholar] [CrossRef]
- Elgadir, M.A.; Uddin, S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, Z.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [CrossRef] [Green Version]
- Safer, A.-M.; Leporatti, S.; Jose, J.; Soliman, M.S. Conjugation Of EGCG And Chitosan NPs As A Novel Nano-Drug Delivery System. Int. J. Nanomed. 2019, 14, 8033–8046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, X.M.; Zheng, F.; Zhang, L.; Miao, Y.Y.; Man, N.; Wen, L.P. Autophagy-mediated chemosensitization by cysteamine in cancer cells. Int. J. Cancer 2011, 129, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Inano, H.; Onoda, M.; Suzuki, K.; Kobayashi, H.; Wakabayashi, K. Inhibitory Effects of WR-2721 and Cysteamine on Tumor Initiation in Mammary Glands of Pregnant Rats by Radiation. Radiat. Res. 2000, 153, 68–74. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X. Synthesis of Chitosan-Stabilized Gold Nanoparticles in the Absence/Presence of Tripolyphosphate. Biomacromolecules 2004, 5, 2340–2346. [Google Scholar] [CrossRef]
- Baptista, P.; Doria, G.; Henriques, D.; Pereira, E.; Franco, R. Colorimetric detection of eukaryotic gene expression with DNA-derivatized gold nanoparticles. J. Biotechnol. 2005, 119, 111–117. [Google Scholar] [CrossRef]
- Coelho, S.C.; Rocha, S.; Juzenas, P.; Sampaio, P.; Almeida, G.M.; Silva, F.S.; Pereira, M.C.; Coelho, M.A.N. Gold nanoparticle delivery-enhanced proteasome inhibitor effect in adenocarcinoma cells. Expert Opin. Drug Deliv. 2013, 10, 1345–1352. [Google Scholar] [CrossRef]
- Abrica-González, P.; Zamora-Justo, J.A.; Sotelo-López, A.; Vázquez-Martínez, G.R.; Balderas-López, J.A.; Muñoz-Diosdado, A.; Ibáñez-Hernández, M. Gold nanoparticles with chitosan, N-acylated chitosan, and chitosan oligosaccharide as DNA carriers. Nanoscale Res. Lett. 2019, 14, 258. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.O.; Gunasekaran, S.; Ravishankar, C.N. Chitosan-capped gold nanoparticles for indicating temperature abuse in frozen stored products. NPJ Sci. Food 2019, 3, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mun, S.; Decker, E.A.; McClements, D.J. Effect of molecular weight and degree of deacetylation of chitosan on the formation of oil-in-water emulsions stabilized by surfactant–chitosan membranes. J. Colloid Interface Sci. 2006, 296, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shi, C.; Tian, L.; Zhu, J. A One-Pot Method to Prepare Gold Nanoparticle Chains with Chitosan. J. Phys. Chem. C 2008, 112, 319–323. [Google Scholar] [CrossRef]
- Jiang, C.; Zhu, J.; Li, Z.; Luo, J.; Wang, J.; Sun, Y. Chitosan–gold nanoparticles as peroxidase mimic and their application in glucose detection in serum. RSC Adv. 2017, 7, 44463–44469. [Google Scholar] [CrossRef] [Green Version]








| Samples | Size, nm | PdI | Zeta Potential, mV |
|---|---|---|---|
| AuNPs | 37 ± 1 | 0.2 | −33 ± 2 |
| CystAuNPs | 54 ± 9 | 0.6 | −37 ± 3 |
| EGCG-CystAuNPs | 111 ± 1 | 0.2 | −24 ± 1 |
| ChAuNPs | 86 ± 16 | 0.2 | 22 ± 5 |
| EGCG-ChAuNPs | 125 ± 13 | 0.3 | 36 ± 6 |
| Antioxidant Activity (mM) | ||||
|---|---|---|---|---|
| Wavelength (nm) | AuChNPs | CystAuNPs | EGCG-ChAuNPs | EGCG-CystAuNPs |
| 750 | - | 1.8 | 2.5 | 3.5 |
| Free EGCG | EGCG-ChAuNPs | EGCG-CystAuNPs | |
|---|---|---|---|
| IC50, μM | 23 ± 3 | 2.22 ± 0.02 | 3.7 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, L.; Coelho, S.C.; Pereira, M.d.C.; Coelho, M.A.N. Nanocarriers Based on Gold Nanoparticles for Epigallocatechin Gallate Delivery in Cancer Cells. Pharmaceutics 2022, 14, 491. https://doi.org/10.3390/pharmaceutics14030491
Cunha L, Coelho SC, Pereira MdC, Coelho MAN. Nanocarriers Based on Gold Nanoparticles for Epigallocatechin Gallate Delivery in Cancer Cells. Pharmaceutics. 2022; 14(3):491. https://doi.org/10.3390/pharmaceutics14030491
Chicago/Turabian StyleCunha, Lídia, Sílvia Castro Coelho, Maria do Carmo Pereira, and Manuel A. N. Coelho. 2022. "Nanocarriers Based on Gold Nanoparticles for Epigallocatechin Gallate Delivery in Cancer Cells" Pharmaceutics 14, no. 3: 491. https://doi.org/10.3390/pharmaceutics14030491