Harnessing the Antibacterial Properties of Fluoridated Chitosan Polymers against Oral Biofilms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fluoridation of Chitosan Polymers and Fluoride Quantification
2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4. Solubility Studies
2.5. In Vitro Inhibition of Acid Demineralisation
2.6. Preparation of Bacterial Cultures
2.7. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.8. Inhibition of S. mutans Biofilm Formation
2.9. Formulation of Chitosan-Based Mouthwash
2.10. Evaluation of Mouthwash Stability
2.11. Biofilm Removal Efficacy
2.12. Cytocompatibility Studies
2.13. Statistical Analysis
3. Results
3.1. Preparation and Characterisation of Fluoridated Polymers
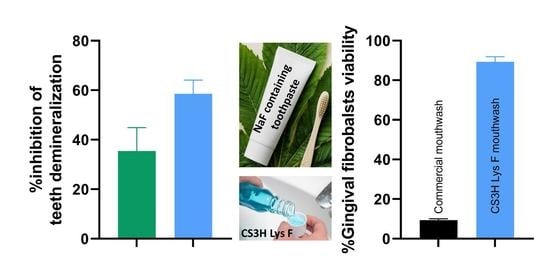
3.2. Inhibition of Acid Demineralisation In Vitro
3.3. Determination of MIC and MBC
3.4. Inhibition of Biofilms Formation
3.5. Cytocompatibility Study
3.6. Stability Studies of Mouthwash Formulations
3.6.1. Organoleptic Properties
3.6.2. Evaluation of pH Stability
3.6.3. Sedimentation
3.6.4. Biofilm Removal Efficacy over Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 11 January 2022).
- Alhabdan, Y.A.; Albeshr, A.G.; Yenugadhati, N.; Jradi, H. Prevalence of dental caries and associated factors among primary school children: A population-based cross-sectional study in Riyadh, Saudi Arabia. Environ. Health Prev. Med. 2018, 23, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.H.; Wong, S.S.S.; Suen, R.P.C.; Lo, E.C.M. Oral health and dental care in Hong Kong. Surgeon 2013, 11, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Duangthip, D.; Gao, S.S.; Lo, E.C.M.; Chu, C.H. Early childhood caries among 5- to 6-year-old children in Southeast Asia. Int. Dent. J. 2017, 67, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandki, R.; Banthia, P.; Banthia, R. Biofilms: A microbial home. J. Indian Soc. Periodontol. 2011, 15, 111–114. [Google Scholar] [CrossRef]
- Nazir, R.; Zaffar, M.R.; Amin, I. Chapter 8—Bacterial Biofilms: The Remarkable Heterogeneous Biological Communities and Nitrogen Fixing Microorganisms in Lakes; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-817495-1. [Google Scholar]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community—Implications for health and disease. BMC Oral Health 2006, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.J.; Ahn, S.J.; Wen, Z.T.; Brady, J.; Burne, R.A. Characteristics of biofilm formation by Streptococcus mutans in the presence of saliva. Infect. Immun. 2008, 76, 4259–4268. [Google Scholar] [CrossRef] [Green Version]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid.-Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- NHS How to Keep Your Teeth Clean. Available online: https://www.nhs.uk/live-well/healthy-body/how-to-keep-your-teeth-clean/ (accessed on 11 January 2022).
- Van Loveren, C.; Buijs, J.F.; Ten Cate, J.M. The effect of triclosan toothpaste on enamel demineralization in a bacterial demineralization model. J. Antimicrob. Chemother. 2000, 45, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Riley, P.; Lamont, T. Triclosan produces statistically significant reduction in plaque, gingivitis and caries but not clinically important benefit. Evid. Based. Dent. 2014, 15, 6–7. [Google Scholar] [CrossRef]
- Quintas, V.; Prada-López, I.; Carreira, M.J.; Suárez-Quintanilla, D.; Balsa-Castro, C.; Tomás, I. In situ antibacterial activity of essential oils with and without alcohol on oral biofilm: A randomized clinical trial. Front. Microbiol. 2017, 8, 2162. [Google Scholar] [CrossRef]
- Vlachojannis, C.; Winsauer, H.; Chrubasik, S. Effectiveness and safety of a mouthwash containing essential oil ingredients. Phytother. Res. 2013, 27, 685–691. [Google Scholar] [CrossRef]
- Sheen, S.; Addy, M. An in vitro evaluation of the availability of cetylpyridinium chloride and chlorhexidine in some commercially available mouthrinse products. Br. Dent. J. 2003, 194, 16–19. [Google Scholar] [CrossRef]
- Mehdipour, M.; Taghavi Zenoz, A.; Asvadi Kermani, I.; Hosseinpour, A. A comparison between zinc sulfate and chlorhexidine gluconate mouthwashes in the prevention of chemotherapy-induced oral mucositis. DARU J. Pharm. Sci. 2011, 19, 71–73. [Google Scholar]
- Najafi, M.H.; Taheri, M.; Mokhtari, M.R.; Forouzanfar, A.; Farazi, F.; Mirzaee, M.; Ebrahiminik, Z.; Mehrara, R. Comparative study of 0.2% and 0.12% digluconate chlorhexidine mouth rinses on the level of dental staining and gingival indices. Dent. Res. J. 2012, 9, 305–308. [Google Scholar]
- Marsh, P.D. Controlling the oral biofilm with antimicrobials. J. Dent. 2010, 38, S11–S15. [Google Scholar] [CrossRef]
- Davison, J.; Maillard, J. Opinion on Triclosan—Antimicrobial Resistance; European Union: Brussels, Belgium, 2010; ISBN 9789279124846. [Google Scholar]
- Storehagen, S.; Ose, N.; Midha, S. Dentifrices and Mouthwashes Ingredients and Their Use. Master’s Thesis, University of Oslo, Oslo, Norway, 2003. [Google Scholar]
- Sreenivasan, P.; Gaffar, A. Antiplaque biocides and bacterial resistance: A review. J. Clin. Periodontol. 2002, 29, 965–974. [Google Scholar] [CrossRef]
- Alencar, M.A.S.D.S.; Martinez, E.F.; Figueiredo, F.C.; De Lima e Silva, A.R.; Protazio, J.E.; Bertamoni, M.; Peruzzo, D.C.; Napimoga, M.H. The Evaluation of Osteoblastic Cell Behavior on Treated Titanium Surface. Open Dent. J. 2019, 14, 1–6. [Google Scholar] [CrossRef]
- Halboub, E.; Al-Maweri, S.A.; Al-Wesabi, M.; Al-Kamel, A.; Shamala, A.; Al-Sharani, A.; Koppolu, P. Efficacy of propolis-based mouthwashes on dental plaque and gingival inflammation: A systematic review. BMC Oral Health 2020, 20, 198. [Google Scholar] [CrossRef]
- Eslami, N.; Ahrari, F.; Rajabi, O.; Zamani, R. The staining effect of different mouthwashes containing nanoparticles on dental enamel. J. Clin. Exp. Dent. 2015, 7, e457–e461. [Google Scholar] [CrossRef]
- Azimi, M.; Jouybari, L.; Moghadam, S.; Ghaemi, E.; Behnampoor, N.; Sanagoo, A.; Hesam, M. Antimicrobial effects of chlorhexidine, matrica drop mouthwash (chamomile extract), and normal saline on hospitalized patients with endotracheal tubes. Iran. J. Nurs. Midwifery Res. 2016, 21, 458. [Google Scholar] [CrossRef] [Green Version]
- Bahlouli, S.; Aghazadeh, Z.; Aghazadeh, M.; Shojani, S.; Kafil, H.S. Determining the Antibacterial Activity of Chlorhexidine Mouthwashes with and without Alcohol against Common Oral Pathogens. J. Adv. Oral Res. 2018, 9, 15–19. [Google Scholar] [CrossRef]
- Vranić, E.; Lacević, A.; Mehmedagić, A.; Uzunović, A. Formulation ingredients for toothpastes and mouthwashes. Bosn. J. Basic Med. Sci. 2004, 4, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Keegan, G.M.; Smart, J.D.; Ingram, M.J.; Barnes, L.M.; Burnett, G.R.; Rees, G.D. Chitosan microparticles for the controlled delivery of fluoride. J. Dent. 2012, 40, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, P.; Nandan, N. Effect of xylitol, sodium fluoride and triclosan containing mouth rinse on Streptococcus mutans. Contemp. Clin. Dent. 2011, 2, 287–290. [Google Scholar] [CrossRef]
- O’Mullane, D.M.; Baez, R.J.; Jones, S.; Lennon, M.A.; Petersen, P.E.; Rugg-Gunn, A.J.; Whelton, H.; Whitford, G.M. Fluoride and Oral Health. Community Dent. Health. 2016, 33, 69–99. [Google Scholar]
- Liao, Y.; Brandt, B.W.; Li, J.; Crielaard, W.; Van Loveren, C.; Mei, D. Fluoride resistance in Streptococcus mutans: A mini review. J. Oral Microbiol. 2017, 9, 1344509. [Google Scholar] [CrossRef] [Green Version]
- Pretty, I.A. High Fluoride Concentration Toothpastes for Children and Adolescents. Caries Res. 2016, 50, 9–14. [Google Scholar] [CrossRef]
- Kavouklis, N.M.; Gaudreault, R.A. Oral Care Formulations with Hydrogen Peroxide and Lycopene. U.S. Patent 11/491,187, 24 July 2006. [Google Scholar]
- Kasaai, M.R.; Arul, J.; Charlet, G. Fragmentation of Chitosan by Acids. Sci. World J. 2013, 2013, 508540. [Google Scholar] [CrossRef] [Green Version]
- Sahariah, P.; Gaware, V.S.; Lieder, R.; Jónsdóttir, S.; Hjálmarsdóttir, M.; Sigurjonsson, O.E.; Másson, M. The effect of substituent, degree of acetylation and positioning of the cationic charge on the antibacterial activity of quaternary chitosan derivatives. Mar. Drugs 2014, 12, 4635–4658. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Rahayu, D.P.; De Mori, A.; Draheim, R.R.; Lalatsa, A.; Roldo, M. Enhancing the antibacterial effect of chitosan to combat orthopaedic implant-associated infections. Carbohydr. Polym. 2021. submitted. [Google Scholar]
- Lalatsa, A.; Garrett, N.L.; Ferrarelli, T.; Moger, J.; Schätzlein, A.G.; Uchegbu, I.F. Delivery of peptides to the blood and brain after oral uptake of quaternary ammonium palmitoyl glycol chitosan nanoparticles. Mol. Pharm. 2012, 9, 1764–1774. [Google Scholar] [CrossRef]
- Kubota, N.; Tatsumoto, N.; Sano, T.; Toya, K. A simple preparation of half N-acetylated chitosan highly soluble in water and aqueous organic solvents. Carbohydr. Res. 2000, 324, 268–274. [Google Scholar] [CrossRef]
- Churchley, D.; Rees, G.D.; Barbu, E.; Nevell, T.G.; Tsibouklis, J. Fluoropolymers as low-surface-energy tooth coatings for oral care. Int. J. Pharm. 2008, 352, 44–49. [Google Scholar] [CrossRef]
- Medina-Flores, D.; Ulloa-Urizar, G.; Camere-Colarossi, R.; Caballero-García, S.; Mayta-Tovalino, F.; del Valle-Mendoza, J. Antibacterial activity of Bixa orellana L. (achiote) against Streptococcus mutans and Streptococcus sanguinis. Asian Pac. J. Trop. Biomed. 2016, 6, 400–403. [Google Scholar] [CrossRef] [Green Version]
- Pasquantonio, G.; Greco, C.; Prenna, M.; Ripa, C.; Vitali, L.A.; Petrelli, D.; Di Luca, M.C.; Ripa, S. Antibacterial activity and anti-biofilm effect of chitosan against strains of Streptococcus mutans isolated in dental plaque. Int. J. Immunopathol. Pharmacol. 2008, 21, 993–997. [Google Scholar] [CrossRef] [Green Version]
- Lemos, J.A.; Abranches, J.; Koo, H.; Marquis, R.E.; Burne, R.A. Protocols to study the physiology of oral biofilms. Methods Mol. Biol. 2010, 666, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Anshula, D.; Rameshwari, R.; Poonacha, K.; Seema, B.; Monika, K.; Neha, P. Evaluation of the Stability, pH, Density and Sedimentation of Green Tea and Green Tea Plus Ginger Mouthwash: A Phytochemical Study. J. Oral Heal. Dent. Sci. 2018, 2. [Google Scholar] [CrossRef]
- Depan, D.; Pesacreta, T.C.; Misra, R.D.K. The synergistic effect of a hybrid graphene oxide-chitosan system and biomimetic mineralization on osteoblast functions. Biomater. Sci. 2014, 2, 264–274. [Google Scholar] [CrossRef]
- Liu, M.; Xu, H.; Ma, Y.; Cheng, J.; Hua, Z.; Huang, G. Osteoblasts proliferation and differentiation stimulating activities of the main components of Epimedii folium. Pharmacogn. Mag. 2017, 13, 90–94. [Google Scholar] [CrossRef]
- Okamoto, H.; Taguchi, H.; Iida, K.; Danjo, K. Development of polymer film dosage forms of lidocaine for buccal administration. J. Control. Release 2001, 77, 253–260. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.M.; Chang, Y.H.; Su, W.W. Modification of different molecular weights of chitosan by p-Coumaric acid: Preparation, characterization and effect of molecular weight on its water solubility and antioxidant property. Int. J. Biol. Macromol. 2019, 136, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Shuai, X.; Unger, F.; Simon, M.; Bi, D.; Kissel, T. The depolymerization of chitosan: Effect on physicochemical and biological properties. Int. J. Pharm. 2004, 281, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, L.; Yan, G.; Chen, F.; Huang, L. Preparation of Drug-loaded Chitosan Microspheres and Its Application in Paper-based PVC Wallpaper. IOP Conf. Ser. Mater. Sci. Eng. 2018, 322. [Google Scholar] [CrossRef]
- Neel, E.A.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization–remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef]
- Burak, A.K.; Özeroğlu, E.; Taspinar, M. The use of methylene blue as mouthwash in periodontology. East. J. Med. 2015, 20, 215–221. [Google Scholar]
- Verma, U.P.; Gupta, A.; Yadav, R.K.; Tiwari, R.; Sharma, R.; Balapure, A.K. Cytotoxicity of chlorhexidine and neem extract on cultured human gingival fibroblasts through fluorescence-activated cell sorting analysis : An in-vitro study. Eur. J. Dent. 2018, 12, 344–349. [Google Scholar] [CrossRef]
- Jain, G.; Khar, R.K.; Ahmad, F.J. Theory and Practice of Physical Pharmacy—E-Book; Elsevier: New Delhi, India, 2012; ISBN 978-81-312-2824-1. [Google Scholar]
- Ojha, S. Formulation and Evaluation of Antibacterial Herbal Mouthwash Against Oral Disorders. Indo Glob. J. Pharm. Sci. 2018, 8, 37–40. [Google Scholar] [CrossRef]
- Hyunh-Ba, K.; Zahn, M. Understanding ICH Guidelines Applicable to Stability Testing. In Handbook of Stability Testing in Pharmaceutical Development: Regulations, Methodologies, and Best Practices; Hyunh-Ba, K., Ed.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Pini, N.I.P.; Lima, D.A.N.L.; Luka, B.; Ganss, C.; Schlueter, N. Viscosity of chitosan impacts the efficacy of F/Sn containing toothpastes against erosive/abrasive wear in enamel. J. Dent. 2020, 92, 103247. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Pina, C.; Tavaria, F.K.; Pintado, M.M. Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens. Anaerobe 2012, 18, 305–309. [Google Scholar] [CrossRef]
- Sahariah, P.; Cibor, D.; Zielińska, D.; Hjálmarsdóttir, M.; Stawski, D.; Másson, M. The effect of molecular weight on the antibacterial activity of N,N,N-trimethyl chitosan (TMC). Int. J. Mol. Sci. 2019, 20, 1743. [Google Scholar] [CrossRef] [Green Version]
- Chow, L.C.; Takagi, S.; Frukhtbeyn, S.; Sieck, B.A.; Parry, E.E.; Liao, N.S.; Schumacher, G.E.; Markovic, M. Remineralization Effect of a Low-Concentration Fluoride Rinse in an Intraoral Model. Caries Res. 2002, 36, 136–141. [Google Scholar] [CrossRef]






| Ingredients | Quantity | Function | |
|---|---|---|---|
| Formula I | Formula II | ||
| Glycerin | 5 g | 5 g | Humectant |
| Sodium saccharin | 450 mg | 450 mg | Sweetener |
| Lutrol 4% w/v | 50 mL | 50 mL | Surfactant |
| Polymer | 0.2 mg | 0.2 mg | Anti-biofilm |
| Acetic acid (0.5 M) | 1 mL | 1 mL | Acidity modifier/co-solvent |
| Food blue | - | 0.2 mL | Coloring agent |
| Peppermint oil | - | 0.25 mL | Flavoring agent |
| Ethanol | - | 20 mL | Co-solvent |
| Water | to 100 mL | to 100 mL | Vehicle |
| Polymers | Transmittance (%) | |||
|---|---|---|---|---|
| pH 6.00 | pH 6.50 | pH 7.00 | pH 7.25 | |
| CS3H | 98.04 ± 0.44 | 97.72 ± 0.13 | 69.15 ± 3.5 | 29.06 ± 3.20 |
| CS3H Flow | 99.34 ± 0.13 ** | 99.51 ± 0.04 *** | 88.32 ± 4.81 ***a | 72.47 ± 5.64 **** |
| CS3H Fmedium | 99.80 ± 0.20 *** | 99.34 ± 0.19 *** | 97.30 ± 1.46 ****ab | 80.21 ± 3.46 **** |
| CS3H Fhigh | 99.52 ± 0.25 *** | 99.46 ± 0.34 *** | 85.80 ± 0.61 ***b | 72.78 ± 3.91 **** |
| CS3H Lys | 92.88 ± 1.07 | 92.71 ± 0.26 | 59.19 ± 7.44 | 25.22 ± 3.34 |
| CS3H Lys F | 88.45 ± 1.89 # | 88.67 ± 1.43 | 86.04 ± 1.16 ## | 48.5 ± 2.42 ### |
| Polymer | Staphylococcus aureus | Streptococcus mutans | ||
|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| CS | 1.60 ± 0.33 | ≥3.0 | 1.50 ± 0.20 | ≥3.0 |
| CS3H | 1.10 ± 0.38 | ≥3.0 | 1.30 ± 0.20 | ≥3.0 |
| CS3H Lys | 1.10 ± 0.60 | ≥3.0 | 1.40 ± 0.23 | ≥3.0 |
| CS3H Lys F | 1.40 ± 0.23 | ≥3.0 | 1.40 ± 0.23 | ≥3.0 |
| Polymer | 25 °C | 40 °C | 25 °C vs. 40 °C |
|---|---|---|---|
| CS | T0 vs. T90 (**) T0 vs. T180 (*) | T0 vs. T3 (*) | ns |
| CS3H | ns | ns | ns |
| CS3H Lys | ns | ns | ns |
| CS3H Lys F | T0 vs. T30 (*) | ns | ns |
| Polymer | Temp | T0 | T30 | T90 | T180 |
|---|---|---|---|---|---|
| CS | 25 °C | 5.54 ± 0.02 | 5.56 ± 0.02 | 5.54 ± 0.02 | 5.53 ± 0.02 |
| 40 °C | 5.53 ± 0.02 | 5.52 ± 0.01 | 5.52 ± 0.03 | ||
| CS3H | 25 °C | 5.52 ± 0.03 | 5.53 ± 0.01 | 5.52 ± 0.01 | 5.52 ± 0.01 |
| 40 °C | 5.50 ± 0.01 | 5.52 ± 0.01 | 5.50 ± 0.01 | ||
| CS3H Lys | 25 °C | 5.52 ± 0.01 | 5.52 ± 0.02 | 5.52 ± 0.02 | 5.52 ± 0.02 |
| 40 °C | 5.48 ± 0.01 * | 5.48 ± 0.03 | 5.48 ± 0.02 | ||
| CS3H Lys F | 25 °C | 5.52 ± 0.02 | 5.54 ± 0.06 | 5.54 ± 0.04 | 5.53 ± 0.05 |
| 40 °C | 5.50 ± 0.04 | 5.52 ± 0.06 | 5.51 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahayu, D.P.; Draheim, R.; Lalatsa, A.; Roldo, M. Harnessing the Antibacterial Properties of Fluoridated Chitosan Polymers against Oral Biofilms. Pharmaceutics 2022, 14, 488. https://doi.org/10.3390/pharmaceutics14030488
Rahayu DP, Draheim R, Lalatsa A, Roldo M. Harnessing the Antibacterial Properties of Fluoridated Chitosan Polymers against Oral Biofilms. Pharmaceutics. 2022; 14(3):488. https://doi.org/10.3390/pharmaceutics14030488
Chicago/Turabian StyleRahayu, Dien Puji, Roger Draheim, Aikaterini Lalatsa, and Marta Roldo. 2022. "Harnessing the Antibacterial Properties of Fluoridated Chitosan Polymers against Oral Biofilms" Pharmaceutics 14, no. 3: 488. https://doi.org/10.3390/pharmaceutics14030488