Printing Drugs onto Nails for Effective Treatment of Onychomycosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Terbinafine HCl Solution ‘Inks’
2.3. Characterization of the Commercial and In-House Prepared Drug Inks
2.3.1. Density
2.3.2. Viscosity
2.3.3. Surface Tension
2.3.4. Scanning Electron Microscope (SEM)
2.4. Calculating Suitable Ink Properties
2.5. High Performance Liquid Chromatography
2.6. Inkjet Printer and Printing Process
2.7. Characterisation of the Prints with X-ray Diffraction (XRD)
2.8. Controlling the Dose of Drug Printed
2.9. In Vitro Antifungal Efficacy
2.9.1. Preparation of Media
2.9.2. Preparation of Fungus Inoculum
2.9.3. Disc Diffusion Assays
2.9.4. Antifungal Susceptibility Testing Using Printed Nail Clippings
3. Results and Discussion
3.1. Printer Optimisation
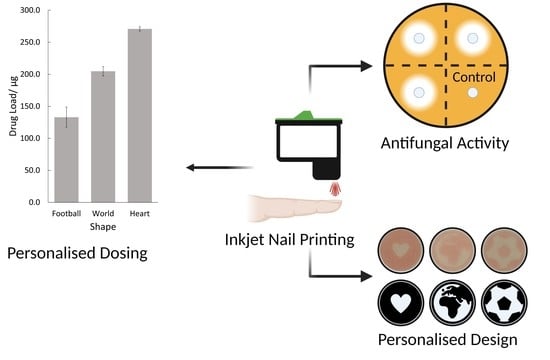
3.2. Personalised Dosing
3.3. In Vitro Antifungal Activity
3.3.1. Disc Diffusion Assay
3.3.2. Nail Diffusion Assay
3.4. General Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scoutaris, N.; Ross, S.; Douroumis, D. Current Trends on Medical and Pharmaceutical Applications of Inkjet Printing Technology. Pharm. Res. 2016, 33, 1799–1816. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhang, D.; Guo, Q.; Xiao, J.; Zheng, M.; Yang, J. Study of the Enzyme Activity Change due to Inkjet Printing for Biosensor Fabrication. ACS Biomater. Sci. Eng. 2021, 7, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Guo, Q.; Xiao, J.; Zheng, M.; Zhang, D.; Yang, J. An inkjet-printed smartphone-supported electrochemical biosensor system for reagentless point-of-care analyte detection. Sens. Actuators B Chem. 2021, 346, 130447. [Google Scholar] [CrossRef]
- Rosati, G.; Urban, M.; Zhao, L.; Yang, Q.; de Carvalho Castro e Silva, C.; Bonaldo, S.; Parolo, C.; Nguyen, E.P.; Ortega, G.; Fornasiero, P.; et al. A plug, print & play inkjet printing and impedance-based biosensing technology operating through a smartphone for clinical diagnostics. Biosens. Bioelectron. 2022, 196, 113737. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ebbens, S.; Zhao, X. Inkjet printing of mammalian cells—Theory and applications. Bioprinting 2021, 23, e00157. [Google Scholar] [CrossRef]
- Saunders, R.E.; Derby, B. Inkjet printing biomaterials for tissue engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- Daly, R.; Harrington, T.S.; Martin, G.D.; Hutchings, I.M. Inkjet printing for pharmaceutics—A review of research and manufacturing. Int. J. Pharm. 2015, 494, 554–567. [Google Scholar] [CrossRef] [Green Version]
- Cader, H.K.; Rance, G.A.; Alexander, M.R.; Gonçalves, A.D.; Roberts, C.J.; Tuck, C.J.; Wildman, R.D. Water-based 3D inkjet printing of an oral pharmaceutical dosage form. Int. J. Pharm. 2019, 564, 359–368. [Google Scholar] [CrossRef]
- Edinger, M.; Bar-Shalom, D.; Sandler, N.; Rantanen, J.; Genina, N. QR encoded smart oral dosage forms by inkjet printing. Int. J. Pharm. 2018, 536, 138–145. [Google Scholar] [CrossRef]
- Fox, C.B.; Nemeth, C.L.; Chevalier, R.W.; Cantlon, J.; Bogdanoff, D.B.; Hsiao, J.C.; Desai, T.A. Picoliter-volume inkjet printing into planar microdevice reservoirs for low-waste, high-capacity drug loading. Bioeng. Transl. Med. 2017, 2, 9–16. [Google Scholar] [CrossRef]
- Uddin, M.J.; Scoutaris, N.; Klepetsanis, P.; Chowdhry, B.; Prausnitz, M.R.; Douroumis, D. Inkjet printing of transdermal microneedles for the delivery of anticancer agents. Int. J. Pharm. 2015, 494, 593–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickström, H.; Palo, M.; Rijckaert, K.; Kolakovic, R.; Nyman, J.O.; Määttänen, A.; Ihalainen, P.; Peltonen, J.; Genina, N.; de Beer, T.; et al. Improvement of dissolution rate of indomethacin by inkjet printing. Eur. J. Pharm. Sci. 2015, 75, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Thabet, Y.; Lunter, D.; Breitkreutz, J. Continuous inkjet printing of enalapril maleate onto orodispersible film formulations. Int. J. Pharm. 2018, 546, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Scoutaris, N.; Chai, F.; Maurel, B.; Sobocinski, J.; Zhao, M.; Moffat, J.G.; Craig, D.Q.; Martel, B.; Blanchemain, N.; Douroumis, D. Development and Biological Evaluation of Inkjet Printed Drug Coatings on Intravascular Stent. Mol. Pharm. 2016, 13, 125–133. [Google Scholar] [CrossRef]
- Tarcha, P.J.; Verlee, D.; Hui, H.W.; Setesak, J.; Antohe, B.; Radulescu, D.; Wallace, D. The Application of Ink-Jet Technology for the Coating and Loading of Drug-Eluting Stents. Ann. Biomed. Eng. 2007, 35, 1791–1799. [Google Scholar] [CrossRef]
- Sandler, N.; Määttänen, A.; Ihalainen, P.; Kronberg, L.; Meierjohann, A.; Viitala, T.; Peltonen, J. Inkjet printing of drug substances and use of porous substrates-towards individualized dosing. J. Pharm. Sci. 2011, 100, 3386–3395. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Monou, P.K.; Bouropoulos, N.; Boetker, J.; Rantanen, J.; Jacobsen, J.; Vizirianakis, I.S.; Fatouros, D.G. Fabrication of Mucoadhesive Buccal Films for Local Administration of Ketoprofen and Lidocaine Hydrochloride by Combining Fused Deposition Modeling and Inkjet Printing. J. Pharm. Sci. 2020, 109, 2757–2766. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Alomari, M.; Dodoo, C.C.; Trenfield, S.J.; Velaga, S.; Basit, A.W.; Gaisford, S. Personalisation of warfarin therapy using thermal ink-jet printing. Eur. J. Pharm. Sci. 2018, 117, 80–87. [Google Scholar] [CrossRef]
- Alomari, M.; Vuddanda, P.R.; Trenfield, S.J.; Dodoo, C.C.; Velaga, S.; Basit, A.W.; Gaisford, S. Printing T3 and T4 oral drug combinations as a novel strategy for hypothyroidism. Int. J. Pharm. 2018, 549, 363–369. [Google Scholar] [CrossRef]
- Dodoo, C.C.; Stapleton, P.; Basit, A.W.; Gaisford, S. The potential of Streptococcus salivarius oral films in the management of dental caries: An inkjet printing approach. Int. J. Pharm. 2020, 591, 119962. [Google Scholar] [CrossRef]
- Azizi Machekposhti, S.; Zhang, B.; Sachan, R.; Vanderwal, L.; Stafslien, S.J.; Narayan, R.J. Patterned surfaces with the controllable drug doses using inkjet printing. J. Mater. Res. 2021, 36, 3865–3876. [Google Scholar] [CrossRef]
- Boehm, R.D.; Daniels, J.; Stafslien, S.; Nasir, A.; Lefebvre, J.; Narayan, R.J. Polyglycolic acid microneedles modified with inkjet-deposited antifungal coatings. Biointerphases 2015, 10, 011004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, R.D.; Jaipan, P.; Skoog, S.A.; Stafslien, S.; VanderWal, L.; Narayan, R.J. Inkjet deposition of itraconazole onto poly(glycolic acid) microneedle arrays. Biointerphases 2016, 11, 011008. [Google Scholar] [CrossRef] [PubMed]
- Boehm, R.D.; Miller, P.R.; Daniels, J.; Stafslien, S.; Narayan, R.J. Inkjet printing for pharmaceutical applications. Mater. Today 2014, 17, 247–252. [Google Scholar] [CrossRef]
- Boehm, R.D.; Miller, P.R.; Schell, W.A.; Perfect, J.R.; Narayan, R.J. Inkjet Printing of Amphotericin B onto Biodegradable Microneedles Using Piezoelectric Inkjet Printing. JOM 2013, 65, 525–533. [Google Scholar] [CrossRef]
- Rich, P.; Scher, R.K. An Atlas of Diseases of the Nail; The Parthenon Publishing Group: London, UK, 2003. [Google Scholar]
- Thomas, J.; Jacobson, G.A.; Narkowicz, C.K.; Peterson, G.M.; Burnet, H.; Sharpe, C. Toenail onychomycosis: An important global disease burden. J. Clin. Pharm. Ther. 2010, 35, 497–519. [Google Scholar] [CrossRef]
- Lipner, S.R.; Scher, R.K. Onychomycosis: Clinical overview and diagnosis. J. Am. Acad. Dermatol. 2019, 80, 835–851. [Google Scholar] [CrossRef]
- NHS. Fungal Nail Infection. Available online: https://www.nhs.uk/conditions/fungal-nail-infection/ (accessed on 1 May 2021).
- Stewart, C.R.; Algu, L.; Kamran, R.; Leveille, C.F.; Abid, K.; Rae, C.; Lipner, S.R. Effect of onychomycosis and treatment on patient-reported quality-of-life outcomes: A systematic review. J. Am. Acad. Dermatol. 2020, 70, 338–351. [Google Scholar] [CrossRef]
- Kayarkatte, M.N.; Singal, A.; Pandhi, D. Impact of Onychomycosis on the Quality of Life: Dermatology Life Quality Index-Based Cross-Sectional Study. Ski. Appendage Disord. 2020, 6, 115–119. [Google Scholar] [CrossRef]
- Elewski, B.E. Onychomycosis. Treatment, quality of life, and economic issues. Am. J. Clin. Dermatol. 2000, 1, 19–26. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. LAMISIL (Terbinafine Hydrochloride) Tablets Label. 1992. Available online: https://www.fda.gov/media/70062/download (accessed on 9 February 2022).
- Ryder, N.S. Terbinafine: Mode of action and properties of the squalene epoxidase inhibition. Br. J. Dermatol. 1992, 126, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Enzo Life Sciences Inc. Terbinafine HCl. 2022. Available online: https://www.enzolifesciences.com/bml-ei318/terbinafine-hcl/ (accessed on 9 February 2022).
- Kuminek, G.; Rauber, G.S.; Riekes, M.K.; Campos, C.E.M.d.; Monti, G.A.; Bortoluzzi, A.J.; Cuffini, S.L.; Cardoso, S.G. Single crystal structure, solid state characterization and dissolution rate of terbinafine hydrochloride. J. Pharm. Biomed. Anal. 2013, 78, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Mordor Intelligence. Onychomycosis Treatment Market-Growth, Trends, COVID-19 Impact, And Forecasts (2021–2026). Online. 2020. Available online: https://www.mordorintelligence.com/industry-reports/onychomycosis-treatment-market (accessed on 13 December 2021).
- Erchonia Corporation. Lunula Laser. Available online: https://www.erchonia.com/products/lunula/ (accessed on 1 May 2021).
- Gupta, A.K.; Versteeg, S.G. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Rovers, J.F.J.; Wagter, L.V.; Greijmans, E.G.E.; Bovenschen, H.J. 1064-nm Nd:YAG laser treatment for onychomycosis: Is it worthwhile? Lasers Med. Sci. 2021, 36, 463–467. [Google Scholar] [CrossRef]
- Ma, W.; Si, C.; Carrero, L.M.K.; Liu, H.-F.; Yin, X.-F.; Liu, J.; Xu, Y.; Zhou, B. Laser treatment for onychomycosis A systematic review and meta-analysis. Medicine 2019, 98, e17948. [Google Scholar] [CrossRef]
- Gupta, A.K.; Ryder, J.E.; Johnson, A.M. Cumulative meta-analysis of systemic antifungal agents for the treatment of onychomycosis. Br. J. Dermatol. 2004, 150, 537–544. [Google Scholar] [CrossRef]
- Shirwaikar, A.A.; Thomas, T.; Shirwaikar, A.; Lobo, R.; Prabhu, K.S. Treatment of onychomycosis: An update. Indian J. Pharm. Sci. 2008, 70, 710–714. [Google Scholar] [CrossRef] [Green Version]
- Murdan, S. Nail disorders in older people, and aspects of their pharmaceutical treatment. Int. J. Pharm. 2016, 512, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Vikas, A.; Rashmin, P.; Mrunali, P.; Chavan, R.B.; Kaushik, T. Mechanistic Insights of Formulation Approaches for the Treatment of Nail Infection: Conventional and Novel Drug Delivery Approaches. AAPS PharmSciTech 2020, 21, 67. [Google Scholar] [CrossRef]
- Shivakumar, H.N.; Juluri, A.; Desai, B.G.; Murthy, S.N. Ungual and Transungual drug delivery. Drug Dev. Ind. Pharm. 2012, 38, 901–911. [Google Scholar] [CrossRef]
- Saner, M.V.; Kulkarni, A.D.; Pardeshi, C.V. Insights into drug delivery across the nail plate barrier. J. Drug Target. 2014, 22, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Aslam, R.; Hussain, T.; Yousaf, A.M.; Ghori, M.U.; Khan, I.U.; Rizvi, S.A.A.; Shahzad, Y. Onychomycosis: Current Understanding and Strategies for Enhancing Drug Delivery into Human Nail Tissue. Curr. Drug Res. Rev. Former. Curr. Drug Abus. Rev. 2021, 13, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.; Gupta, A.K.; Versteeg, S.; Mays, R.; Villanueva, E.; John, D. Topical and device-based treatments for fungal infections of the toenails. Cochrane Database Syst. Rev. 2020, CD012093. [Google Scholar] [CrossRef]
- Rizi, K.; Mohammed, I.K.; Xu, K.; Kinloch, A.J.; Charalambides, M.N.; Murdan, S. A systematic approach to the formulation of anti-onychomycotic nail patches. Eur. J. Pharm. Biopharm. 2018, 127, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Kerai, L.V.; Hilton, S.; Murdan, S. UV-curable gel formulations: Potential drug carriers for the topical treatment of nail diseases. Int. J. Pharm. 2015, 492, 177–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerai, L.V.; Hilton, S.; Maugueret, M.; Kazi, B.B.; Faull, J.; Bhakta, S.; Murdan, S. UV-curable gels as topical nail medicines:In vivo residence, anti-fungal efficacy and influence of gel components on their properties. Int. J. Pharm. 2016, 514, 244–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerai, L.V.; Bardés, J.; Hilton, S.; Murdan, S. Two strategies to enhance ungual drug permeation from UV-cured films: Incomplete polymerisation to increase drug release and incorporation of chemical enhancers. Eur. J. Pharm. Sci. 2018, 123, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, R.; Targhotra, M.; Kumar, B.; Sahoo, P.K.; Chauhan, M.K. Novel Polypseudorotaxanes Hydrogel based Nail Lacquer of Efinaconazole for Transungual Drug Delivery. Drug Deliv. Lett. 2021, 11, 52–61. [Google Scholar] [CrossRef]
- Souza, A.M.S.; Ribeiro, R.C.A.; Pinheiro, G.K.L.O.; Pinheiro, F.I.; Oliveira, W.N.; Souza, L.B.F.C.; Silva, A.L.; Amaral-Machado, L.; Alencar, É.N.; Chaves, G.M.; et al. Polishing the Therapy of Onychomycosis Induced by Candida spp.: Amphotericin B–Loaded Nail Lacquer. Pharmaceutics 2021, 13, 784. [Google Scholar] [CrossRef]
- Rahman, A.; Aqil, M.; Ahad, A.; Imam, S.S.; Qadir, A.; Ali, A. Application of central composite design for the optimization of itraconazole loaded nail lacquer formulation. 3 Biotech 2021, 11, 324. [Google Scholar] [CrossRef]
- Gupta, A.K.; Foley, K.A. Evidence for biofilms in onychomycosis. G Ital. Dermatol. Venereol. 2019, 154, 50–55. [Google Scholar] [CrossRef]
- Pereira, D.L.; Vila, T.; Borba-Santos, P.L.; de Souza, W.; Navarro, M.; Rozental, S. Activity of Metal-Azole Complexes Against Biofilms of Candida albicans and Candida glabrata. Curr. Pharm. Des. 2020, 26, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Iozumi, K.; Abe, M.; Ito, Y.; Uesugi, T.; Onoduka, T.; Kato, I.; Kato, F.; Kodama, K.; Takahashi, H.; Takeda, O.; et al. Efficacy of long-term treatment with efinaconazole 10% solution in patients with onychomycosis, including severe cases: A multicenter, single-arm study. J. Dermatol. 2019, 46, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M. Characteristics and Efficacy of Two Topical Therapeutic Agents for Onychomycosis Efinaconazole 10% Solution and Luliconazole 5% Solution. Med Mycol. J. 2019, 60, 71–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elewski, B.E.; Tosti, A.; Lin, T. Efinaconazole 10% Topical Solution: Case Review of Onychomycosis Patients Who Were Completely Cured at Week 24. Ski. Appendage Disord. 2018, 4, 67–70. [Google Scholar] [CrossRef]
- Fromm, J.E. Numerical Calculation of the Fluid Dynamics of Drop-on-Demand Jets. IBM J. Res. Dev. 1984, 28, 322–333. [Google Scholar] [CrossRef]
- Jang, D.; Kim, D.; Moon, J. Influence of Fluid Physical Properties on Ink-Jet Printability. Langmuir 2009, 25, 2629–2635. [Google Scholar] [CrossRef]
- Derby, B.; Reis, N. Inkjet Printing of Highly Loaded Particulate Suspensions. MRS Bull. 2003, 28, 815–818. [Google Scholar] [CrossRef]
- Khattab, I.S.; Bandarkar, F.; Fakhree, M.A.A.; Jouyban, A. Density, viscosity, and surface tension of water+ethanol mixtures from 293 to 323K. Korean J. Chem. Eng. 2012, 29, 812–817. [Google Scholar] [CrossRef]
- Meléndez, P.A.; Kane, K.M.; Ashvar, C.S.; Albrecht, M.; Smith, P.A. Thermal Inkjet Application in the Preparation of Oral Dosage Forms: Dispensing of Prednisolone Solutions and Polymorphic Characterization by Solid-State Spectroscopic Techniques. J. Pharm. Sci. 2008, 97, 2619–2636. [Google Scholar] [CrossRef]
- Thatai, P.; Sapra, B. Terbinafine hydrochloride nail lacquer for the management of onychomycosis: Formulation, characterization and in vitro evaluation. Ther. Deliv. 2018, 9, 99–119. [Google Scholar] [CrossRef]
- Singh, J.; Zaman, M.; Gupta, A.K. Evaluation of microdilution and disk diffusion methods for antifungal susceptibility testing of dermatophytes. Med. Mycol. 2007, 45, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Rizi, K.; Xu, K.; Begum, T.; Faull, J.; Bhakta, S.; Murdan, S. A drug-in-adhesive anti-onychomycotic nail patch: Influence of drug and adhesive nature on drug release, ungual permeation, in vivo residence in human and anti-fungal efficacy. Int. J. Pharm. 2022, 614, 121437. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Gorain, B.; Jacob, S.; Attimarad, M.; Sreeharsha, N.; Venugopala, K.N.; Morsy, M.A. Constant Voltage Iontophoresis Technique to Deliver Terbinafine via Transungual Delivery System: Formulation Optimization Using Box–Behnken Design and In Vitro Evaluation. Pharmaceutics 2021, 13, 1692. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Lewis, R.E. Toward more effective antifungal therapy: The prospects of combination therapy. Br. J. Haematol. 2004, 126, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.; Khurana, A. An update on treatment of onychomycosis. Mycoses 2012, 55, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Gunt, H.B.; Kasting, G.B. Effect of hydration on the permeation of ketoconazole through human nail plate in vitro. Eur. J. Pharm. Sci. 2007, 32, 254–260. [Google Scholar] [CrossRef]
- Abobakr, F.E.; Fayez, S.M.; Elwazzan, V.S.; Sakran, W. Effect of Different Nail Penetration Enhancers in Solid Lipid Nanoparticles Containing Terbinafine Hydrochloride for Treatment of Onychomycosis. AAPS PharmSciTech 2021, 22, 33. [Google Scholar] [CrossRef]






| Solution | Density (g cm−3) | Viscosity (mPa·s−1) | Surface Tension (mN·m−1) | Z Value |
|---|---|---|---|---|
| Yellow Commercial Ink | 1.052 | 1.99 | 35.70 | 14.11 |
| Magenta Commercial Ink | 1.03 | 2.24 | 35.63 | 12.39 |
| Cyan Commercial Ink | 1.00 | 2.28 | 35.50 | 11.98 |
| Ethanol and Water | 0.828 | 1.97 | 26.33 | 10.86 |
| Ethanol, Water and 1 mg/mL Terbinafine HCl | 0.831 | 1.95 | 26.30 | 10.99 |
| Ethanol, Water, 1 mg/mL Terbinafine HCl and Food Colouring | 0.809 | 2.00 | 26.37 | 10.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollard, T.D.; Bonetti, M.; Day, A.; Gaisford, S.; Orlu, M.; Basit, A.W.; Murdan, S.; Goyanes, A. Printing Drugs onto Nails for Effective Treatment of Onychomycosis. Pharmaceutics 2022, 14, 448. https://doi.org/10.3390/pharmaceutics14020448
Pollard TD, Bonetti M, Day A, Gaisford S, Orlu M, Basit AW, Murdan S, Goyanes A. Printing Drugs onto Nails for Effective Treatment of Onychomycosis. Pharmaceutics. 2022; 14(2):448. https://doi.org/10.3390/pharmaceutics14020448
Chicago/Turabian StylePollard, Thomas D., Margherita Bonetti, Adam Day, Simon Gaisford, Mine Orlu, Abdul W. Basit, Sudaxshina Murdan, and Alvaro Goyanes. 2022. "Printing Drugs onto Nails for Effective Treatment of Onychomycosis" Pharmaceutics 14, no. 2: 448. https://doi.org/10.3390/pharmaceutics14020448