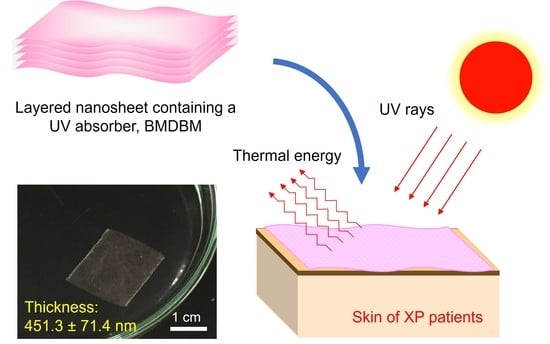
Potential UV-Protective Effect of Freestanding Biodegradable Nanosheet-Based Sunscreen Preparations in XPA-Deficient Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of BMDBM Nanosheet Preparations
2.1.1. Materials
2.1.2. Fabrication of Nanosheets and BMDBM Nanosheets
2.1.3. Fabrication of Layered Nanosheets and BMDBM-Layered Nanosheets
2.2. Morphology of BMDBM Nanosheet Preparations
2.2.1. Macroscopic Morphology
2.2.2. Microscopic Morphology
2.2.3. Measurement of Thickness
2.3. Optical Analysis of the BMDBM Nanosheet Preparations
2.3.1. Ultraviolet Visible Light Absorption (UV–Vis) Spectroscopy
2.3.2. Fourier Transform Infrared (FT-IR) Spectroscopy
2.4. Photoprotective Ability of the BMDBM Nanosheet Preparations
2.4.1. Mice
2.4.2. UV Irradiation
2.4.3. Observation of Inflammatory Reactions
2.4.4. Histological Analysis
2.4.5. Statistics
3. Results
3.1. BMDBM Nanosheet Preparations
3.1.1. BMDBM Nanosheets
3.1.2. BMDBM-Layered Nanosheets
3.2. UV Protective Effect in XPA-Deficient Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DiGiovanna, J.J.; Kraemer, K.H. Shining a light on xeroderma pigmentosum. J. Investig. Dermatol. 2012, 132, 785–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, J.O. Xeroderma pigmentosum. Head Neck Pathol. 2016, 10, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Nishigori, C.; Nakano, E.; Masaki, T.; Ono, R.; Takeuchi, S.; Tsujimoto, M.; Ueda, T. Characteristics of xeroderma pigmentosum in Japan: Lessons from two clinical surveys and measures for patient care. Photochem. Photobiol. 2019, 95, 140–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleaver, J.E. Defective repair replication of DNA in xeroderma pigmentosum. Nature 1968, 218, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.T.; Goldstein, A.M.; Tamura, D.; Khan, S.G.; Ueda, T.; Boyle, J.; Oh, K.S.; Imoto, K.; Inui, H.; Moriwaki, S.; et al. Cancer and neurologic degeneration in xeroderma pigmentosum: Long term follow-up characterises the role of DNA repair. J. Med. Genet. 2011, 48, 168–176. [Google Scholar] [CrossRef]
- Masutani, C.; Kusumoto, R.; Yamada, A.; Dohmae, N.; Yokoi, M.; Yuasa, M.; Araki, M.; Iwai, S.; Takio, K.; Hanaoka, F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase η. Nature 1999, 399, 700–704. [Google Scholar] [CrossRef]
- Hirai, Y.; Kodama, Y.; Moriwaki, S.; Noda, A.; Cullings, H.M.; Macphee, D.G.; Kodama, K.; Mabuchi, K.; Kraemer, K.H.; Land, C.E.; et al. Heterozygous individuals bearing a founder mutation in the XPA DNA repair gene comprise nearly 1% of the Japanese population. Mutat. Res. 2006, 601, 171–178. [Google Scholar] [CrossRef]
- Tamura, D.; DiGiovanna, J.J.; Khan, S.G.; Kraemer, K.H. Living with xeroderma pigmentosum: Comprehensive photoprotection for highly photosensitive patients. Photodermatol. Photoimmunol. Photomed. 2014, 30, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Walburn, J.; Canfield, M.; Norton, S.; Sainsbury, K.; Araújo-Soares, V.; Foster, L.; Berneburg, M.; Sarasin, A.; Morrison-Bowen, N.; Sniehotta, F.F.; et al. Psychological correlates of adherence to photoprotection in a rare disease: International survey of people with xeroderma pigmentosum. Br. J. Health Psychol. 2019, 24, 668–686. [Google Scholar] [CrossRef]
- Azurdia, R.M.; Pagliaro, J.A.; Diffey, B.L.; Rhodes, L.E. Sunscreen application by photosensitive patients is inadequate for protection. Br. J. Dermatol. 1999, 140, 255–258. [Google Scholar] [CrossRef]
- Yang, H.P.; Chen, K.; Ju, M.; Chang, B.Z.; Wang, L.Y.; Gu, H. A study of the way in which dermatologists and photosensitive patients apply sunscreen in China. Photodermatol. Photoimmunol. Photomed. 2009, 25, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.; Datta, P.; Philipsen, P.A.; Wulf, H.C. Sunscreen use and failures-on site observations on a sun-holiday. Photochem. Photobiol. Sci. 2013, 12, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Bhushan, B.; Ge, S. Friction, adhesion and durability and influence of humidity on adhesion and surface charging of skin and various skin creams using atomic force microscopy. J. Microsc. 2010, 239, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugibayashi, K. Skin Permeation and Disposition of Therapeutic and Cosmeceutical Compounds, 1st ed.; Springer: Tokyo, Japan, 2017; pp. 111–161. [Google Scholar]
- Kado, Y.; Mitsuishi, M.; Miyashita, T. Fabrication of three-dimensional nanostructures using reactive polymer nanosheets. Adv. Mater. 2005, 17, 1857–1861. [Google Scholar] [CrossRef]
- Cheng, W.; Campolongo, M.J.; Tan, S.J.; Luo, D. Freestanding ultrathin nano-membranes via self-assembly. Nano Today 2009, 4, 482–493. [Google Scholar] [CrossRef]
- Okamura, Y.; Kabata, K.; Kinoshita, M.; Saitoh, D.; Takeoka, S. Free-standing biodegradable poly(lactic acid) nanosheet for sealing operations in surgery. Adv. Mater. 2009, 21, 4388–4392. [Google Scholar] [CrossRef]
- Fujie, T.; Okamura, Y.; Takeoka, S. Ubiquitous Transference of a free-standing polysaccharide nanosheet with the development of a nano-adhesive plaster. Adv. Mater. 2007, 19, 3549–3553. [Google Scholar] [CrossRef]
- Huang, K.C.; Yano, F.; Murahashi, Y.; Takano, S.; Kitaura, Y.; Chang, S.H.; Ueng, S.W.N.; Tanaka, S.; Ishihara, K.; Okamura, Y.; et al. Sandwich-type PLLA-nanosheets loaded with BMP-2 induce bone regeneration in critical-sized mouse calvarial defects. Acta Biomater. 2017, 59, 12–20. [Google Scholar] [CrossRef]
- Murahashi, Y.; Yano, F.; Nakamoto, H.; Maenohara, Y.; Iba, K.; Yamashita, T.; Tanaka, S.; Ishihara, K.; Okamura, Y.; Moro, T.; et al. Multi-layered PLLA-nanosheets loaded with FGF-2 induce robust bone regeneration with controlled release in critical-sized mouse femoral defects. Acta Biomater. 2019, 85, 172–179. [Google Scholar] [CrossRef]
- Hatanaka, T.; Saito, T.; Fukushima, T.; Todo, H.; Sugibayashi, K.; Umehara, S.; Takeuchi, T.; Okamura, Y. Potential of biocompatible polymeric ultra-thin films, nanosheets, as topical and transdermal drug delivery devices. Int. J. Pharm. 2019, 565, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Komachi, T.; Sumiyoshi, H.; Inagaki, Y.; Takeoka, S.; Nagase, Y.; Okamura, Y. Adhesive and robust multilayered poly(lactic acid) nanosheets for hemostatic dressing in liver injury model. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Nakane, H.; Takeuchi, S.; Yuba, S.; Saijo, M.; Nakatsu, Y.; Murai, H.; Nakatsuru, Y.; Ishikawa, T.; Hirota, S.; Kitamura, Y.; et al. High incidence of ultraviolet-B-or chemical-carcinogen-induced skin tumors in mice lacking the xeroderma pigmentosum group A gene. Nature 1995, 377, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sunami, Y.; Hashimoto, H. Mini Review: Nanosheet technology towards biomedical application. Nanomaterials 2017, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Ghomi, E.R.; Khosravi, F.; Ardahaei, A.S.; Dai, Y.; Neisiany, R.E.; Foroughi, F.; Wu, M.; Das, O.; Ramakrishna, S. The life cycle assessment for polylactic acid (PLA) to make it a low-carbon material. Polymers 2021, 13, 1854. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Husárová, L.; Pekařová, S.; Stloukal, P.; Kucharzcyk, P.; Verney, V.; Commereuc, S.; Ramone, A.; Koutny, M. Identification of important abiotic and biotic factors in the biodegradation of poly(l-lactic acid). Int. J. Biol. Macromol. 2014, 71, 155–162. [Google Scholar] [CrossRef]
- Huang, Q.; Hiyama, M.; Kabe, T.; Kimura, S.; Iwata, T. Enzymatic self-biodegradation of poly(l-lactic acid) films by embedded heat-treated and immobilized proteinase K. Biomacromolecules 2020, 10, 3301–3307. [Google Scholar] [CrossRef]
- Cho, J.; Char, K.; Hong, J.D.; Lee, K.B. Fabrication of highly ordered multilayer films using a spin self-assembly method. Adv. Mater. 2001, 13, 1076–1078. [Google Scholar] [CrossRef]
- Mattsson, J.; Forrest, J.A.; Börjesson, L. Quantifying glass transition behavior in ultrathin free-standing polymer films. Phys. Rev. E 2000, 62, 5187–5200. [Google Scholar] [CrossRef]
- Horio, T.; Miyauchi-Hashimoto, H.; Kuwamoto, K.; Yamazaki, F.; Okamoto, H. Photobiological information obtained from XPA gene-deficient mice. Photochem. Photobiol. 2007, 83, 218–224. [Google Scholar] [PubMed]
- Kunisada, M.; Yamano, N.; Hosaka, C.; Takemori, C.; Nishigori, C. Inflammation due to voriconazole-induced photosensitivity enhanced skin phototumorigenesis in Xpa-knockout mice. Photochem. Photobiol. 2018, 94, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Ono, R.; Masaki, T.; Dien, S.; Yu, X.; Fukunaga, A.; Yodoi, J.; Nishigori, C. Suppressive effect of recombinant human thioredoxin on ultraviolet light-induced inflammation and apoptosis in murine skin. J. Dermatol. 2012, 39, 843–851. [Google Scholar] [CrossRef]
- Yogianti, F.; Kunisada, M.; Nakano, E.; Ono, R.; Sakumi, K.; Oka, S.; Nakabeppu, Y.; Nishigori, C. Inhibitory effects of dietary Spirulina platensis on UVB-induced skin inflammatory responses and carcinogenesis. J. Investig. Dermatol. 2014, 134, 2610–2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, B.; Wulf, H.C. Application of sunscreen-theory and reality. Photodermatol. Photoimmunol. Photomed. 2014, 30, 96–101. [Google Scholar] [CrossRef]




| Preparation | Building Blocks | Sacrificial Layers | Final Thickness e (nm) | ||
|---|---|---|---|---|---|
| PLLA a (% w/v) | BMDBM b (% w/v) | PVA c (% w/v) | Na-Alg d (% w/v) | ||
| Nanosheet | 1.0 | 1.0 | 58.7 ± 4.1 | ||
| BMDBM nanosheet | 1.0 | 0.5 | 1.0 | 77.0 ± 1.8 | |
| Layered nanosheet | 1.0 | 1.8 | 2.5 | 360.0 ± 4.0 | |
| BMDBM-layered nanosheet | 1.0 | 0.5 | 1.8 | 2.5 | 451.3 ± 71.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatanaka, T.; Ramphai, K.; Takimoto, S.; Kanda, H.; Motosugi, N.; Kimura, M.; Mabuchi, T.; Oyama, M.; Takeuchi, T.; Okamura, Y. Potential UV-Protective Effect of Freestanding Biodegradable Nanosheet-Based Sunscreen Preparations in XPA-Deficient Mice. Pharmaceutics 2022, 14, 431. https://doi.org/10.3390/pharmaceutics14020431
Hatanaka T, Ramphai K, Takimoto S, Kanda H, Motosugi N, Kimura M, Mabuchi T, Oyama M, Takeuchi T, Okamura Y. Potential UV-Protective Effect of Freestanding Biodegradable Nanosheet-Based Sunscreen Preparations in XPA-Deficient Mice. Pharmaceutics. 2022; 14(2):431. https://doi.org/10.3390/pharmaceutics14020431
Chicago/Turabian StyleHatanaka, Tomomi, Khampeeraphan Ramphai, Shun Takimoto, Hiromi Kanda, Nami Motosugi, Minoru Kimura, Tomotaka Mabuchi, Midori Oyama, Tomoharu Takeuchi, and Yosuke Okamura. 2022. "Potential UV-Protective Effect of Freestanding Biodegradable Nanosheet-Based Sunscreen Preparations in XPA-Deficient Mice" Pharmaceutics 14, no. 2: 431. https://doi.org/10.3390/pharmaceutics14020431