The Hitchhiker’s Guide to Human Therapeutic Nanoparticle Development
Abstract
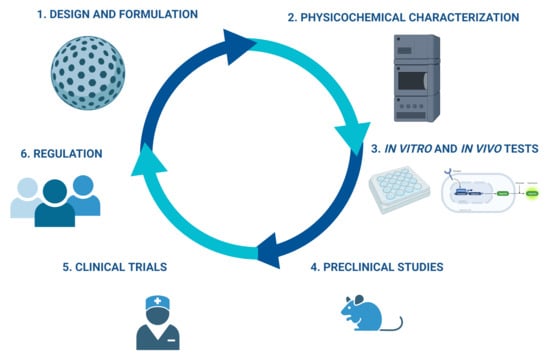
:1. Nanoparticles in Medicine
2. First Approaches of Regulatory Agencies in Nanoparticle Development
3. Challenges in the Design and Physicochemical Characterization of Nanoformulations
3.1. Physicochemical Characterization
3.2. Regulatory Aspects of the Physicochemical Characterization of Nps
4. Challenges for In Vitro Biological Evaluation
4.1. In Vitro Assays: Cell Lines
4.2. Characterization in Biological Fluids
4.3. Toxicity in In Vitro Models
4.4. Regulation of In Vitro Models in NPs
5. In Vivo Tests
5.1. Selection of Animal Models and Test Parameters
5.2. Types of Preclinical Studies for NPs
5.3. Pharmacokinetics (PK) of NPs
5.4. Immunotoxicity of NPs
5.5. Regulation of In Vivo Assays on NPs
5.5.1. Regulation of Pharmacokinetic Studies
5.5.2. Regulation of Immunotoxicity
6. Process Manufacturing
6.1. Stability
6.2. Scalability
6.3. Regulations in the Manufacturing Process
7. Clinical Trials
7.1. Clinical Trial Regulations
7.2. Regulation for NPs in Latin America
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Q.; Wang, H. New era of drug innovation in China. Acta Pharm. Sin. B 2019, 9, 1084. [Google Scholar] [CrossRef] [PubMed]
- Lembo, D.; Donalisio, M.; Civra, A.; Argenziano, M.; Cavalli, R. Nanomedicine formulations for the delivery of antiviral drugs: A promising solution for the treatment of viral infections. Expert Opin. Drug Deliv. 2017, 15, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. New Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertig, J.B.; Shah, V.P.; Flühmann, B.; Mühlebach, S.; Stemer, G.; Surugue, J.; Moss, R.; Di Francesco, T. Tackling the challenges of nanomedicines: Are we ready? Am. J. Health Pharm. 2021, 78, 1047–1056. [Google Scholar] [CrossRef]
- Theek, B.; Rizzo, L.Y.; Ehling, J.; Kiessling, F.; Lammers, T. The theranostic path to personalized nanomedicine. Clin. Transl. Imaging 2014, 2, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef]
- Laksee, S.; Sansanaphongpricha, K.; Puthong, S.; Sangphech, N.; Palaga, T.; Muangsin, N. New organic/inorganic nanohybrids of targeted pullulan derivative/gold nanoparticles for effective drug delivery systems. Int. J. Biol. Macromol. 2020, 162, 561–577. [Google Scholar] [CrossRef]
- Thakore, S.I.; Solanki, A.; Das, M. Chapter 4—Exploring potential of polymers in cancer management. In Materials for Biomedical Engineering; Holban, A.-M., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–133. [Google Scholar]
- Zununi Vahed, S.; Salehi, R.; Davaran, S.; Sharifi, S. Liposome-based drug co-delivery systems in cancer cells. Mater. Sci. Eng. C 2017, 71, 1327–1341. [Google Scholar] [CrossRef]
- Amin, M.C.I.M.; Butt, A.M.; Amjad, M.W.; Kesharwani, P. Chapter 5—Polymeric Micelles for Drug Targeting and Delivery. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Mishra, V., Kesharwani, P., Mohd Amin, M.C.I., Iyer, A., Eds.; Academic Press: Cambrigde, MA, USA, 2017; pp. 167–202. [Google Scholar]
- Silva, E.K.; Meireles, M.A.A. Encapsulation of Food Compounds Using Supercritical Technologies: Applications of Supercritical Carbon Dioxide as an Antisolvent. Food Public Health 2014, 4, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Ramos, T.I.; Villacis-Aguirre, C.A.; Vispo, N.S.; Padilla, L.S.; Santana, S.P.; Parra, N.C.; Alonso, J.R.T. Forms and Methods for Interferon’s Encapsulation. Pharmaceutics 2021, 13, 1533. [Google Scholar] [CrossRef]
- Yasukawa, T.; Ogura, Y.; Tabata, Y.; Kimura, H.; Wiedemann, P.; Honda, Y. Drug delivery systems for vitreoretinal diseases. Prog. Retin. Eye Res. 2004, 23, 253–281. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Saifullah, M.; Shishir, M.R.I.; Ferdowsi, R.; Tanver Rahman, M.R.; Van Vuong, Q. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.L.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Yun, Y.; Park, K. PLA micro- and nano-particles. Adv. Drug Deliv. Rev. 2016, 107, 176–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeinzadeh, S.; Jabbari, E. Nanoparticles and Their Applications. In Springer Handbook of Nanotechnology; Bhushan, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 335–361. [Google Scholar]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef]
- Kehoe, T.; Blind, E.; Janssen, H. Regulatory aspects of the development of drugs for metabolic bone diseases—FDA and EMA perspective. Br. J. Clin. Pharmacol. 2018, 85, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.; Belz, S.; Hoeveler, A.; Hugas, M.; Okuda, H.; Patri, A.; Rauscher, H.; Silva, P.; Slikker, W.; Sokull-Kluettgen, B.; et al. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul. Toxicol. Pharmacol. 2021, 122, 104885. [Google Scholar] [CrossRef] [PubMed]
- Tesauro, D.; Accardo, A.; Aloj, L.; Morelli, G.; Aurilio, M. Receptor binding peptides for target-selective delivery of nanoparticles encapsulated drugs. Int. J. Nanomed. 2014, 9, 1537–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The regulation of nanomaterials and nanomedicines for clinical application: Current and future perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef] [PubMed]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, M.R.; Choo, Q.; Ashtikar, M.; Rocha, T.C.; Bremer-Hoffmann, S.; Wacker, M.G. Nanomedicines—Tiny particles and big challenges. Adv. Drug Deliv. Rev. 2019, 151–152, 23–43. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv. Drug Deliv. Rev. 2020, 154-155, 37–63. [Google Scholar] [CrossRef]
- Kargul, J.; Irminger-Finger, I.; Laurent, G.J. Nanomedicine: Application of nanoparticles in clinical therapies and diagnostics. Int. J. Biochem. Cell Biol. 2016, 75, 140. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Pearce, A.K.; O’Reilly, R.K. Insights into Active Targeting of Nanoparticles in Drug Delivery: Advances in Clinical Studies and Design Considerations for Cancer Nanomedicine. Bioconjugate Chem. 2019, 30, 2300–2311. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, U.A.; Riaz, M.; Yasmeen, E.; Yousaf, M.Z. Recent Advances in Nanoparticle-Based Targeted Drug-Delivery Systems Against Cancer and Role of Tumor Microenvironment. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 317–353. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Alsowinea, A.F. Approved and marketed nanoparticles for disease targeting and applications in COVID-19. Nanotechnol. Rev. 2021, 10, 1941–1977. [Google Scholar] [CrossRef]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging carriers for drug delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; De Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2012, 71, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Guo, D.; Jiang, Z.; Tong, R.; Jiang, P.; Bai, L.; Chen, L.; Zhu, Y.; Guo, C.; Shi, J.; et al. Novel HER2-Targeting Antibody-Drug Conjugates of Trastuzumab Beyond T-DM1 in Breast Cancer: Trastuzumab Deruxtecan(DS-8201a) and (Vic-)Trastuzumab Duocarmazine (SYD985). Eur. J. Med. Chem. 2019, 183, 111682. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Glover, R.E.; Urquhart, R.; Lukawska, J.; Blumenthal, K.G. Vaccinating against Covid-19 in people who report allergies. BMJ 2021, 372, n120. [Google Scholar] [CrossRef]
- Hotez, P.J.; Nuzhath, T.; Callaghan, T.; Colwell, B. COVID-19 vaccine decisions: Considering the choices and opportunities. Microbes Infect. 2021, 23, 104811. [Google Scholar] [CrossRef]
- Hansen, R.B.; Kavanaugh, A. Certolizumab pegol for the treatment of psoriatic arthritis. Expert Rev. Clin. Immunol. 2015, 11, 307–318. [Google Scholar] [CrossRef]
- Teixeira, T.; Kweder, S.L.; Saint-Raymond, A. Are the European Medicines Agency, US Food and Drug Administration, and Other International Regulators Talking to Each Other? Clin. Pharmacol. Ther. 2019, 107, 507–513. [Google Scholar] [CrossRef]
- Halamoda-Kenzaoui, B.; Box, H.; van Elk, M.; Gaitan, S.; Geertsma, R.E.; Gainza Lafuente, E.; Owen, A.; Del Pozo, A.; Roesslein, M.; Bremer-Hoffmann, S. Anticipation of Regulatory Needs for Nanotechnology-Enabled Health Products: The REFINE White Paper; Publications Office of the European Union: Luxembourg, 2019.
- Gioria, S.; Caputo, F.; Urbán, P.; Maguire, C.M.; Bremer-Hoffmann, S.; Prina-Mello, A.; Calzolai, L.; Mehn, D. Are existing standard methods suitable for the evaluation of nanomedicines: Some case studies. Nanomedicine 2018, 13, 539–554. [Google Scholar] [CrossRef] [Green Version]
- Agrahari, V.; Agrahari, V. Facilitating the translation of nanomedicines to a clinical product: Challenges and opportunities. Drug Discov. Today 2018, 23, 974–991. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Alzahrani, N.; Alzahrani, R.; Alshamrani, W.; Aloufi, W.; Ali, A.; Najib, S.; Siddiqui, N.A. Stability issues and approaches to stabilised nanoparticles based drug delivery system. J. Drug Target. 2020, 28, 468–486. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Hackley, V.A.; Prina-Mello, A.; Puri, S.; Sonzini, S.; Soo, P.L. Sizing up the next generation of nanomedicines. Pharm. Res. 2019, 37, 6. [Google Scholar] [CrossRef] [PubMed]
- Bisso, S.; Leroux, J.-C. Nanopharmaceuticals: A focus on their clinical translatability. Int. J. Pharm. 2020, 578, 119098. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; McNeil, S.E. In Vitro Assays for Monitoring Nanoparticle Interaction with Components of the Immune System. In Handbook of Immunological Properties of Engineered Nanomaterials, Frontiers in Nanobiomedical Research; World Scientific: Singapore, 2016; Volume 6, pp. 223–280. [Google Scholar]
- Neun, B.W.; Dobrovolskaia, M.A. Considerations and some practical solutions to overcome nanoparticle interference with LAL assays and to avoid endotoxin contamination in nanoformulations. Methods Mol. Biol. 2018, 1682, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Irby, D.; Du, C.; Li, F. Lipid–drug conjugate for enhancing drug delivery. Mol. Pharm. 2017, 14, 1325–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyhan, A.A. Lost in translation: The valley of death across preclinical and clinical divide—Identification of problems and overcoming obstacles. Transl. Med. Commun. 2019, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashoki, M.; Hanaizi, Z.; Yordanova, S.; Veselý, R.; Bouygues, C.; Llinares, J.; Kweder, S.L. A Comparison ofEMAandFDADecisions for new drug marketing applications 2014–2016: Concordance, discordance, and why. Clin. Pharmacol. Ther. 2019, 107, 195–202. [Google Scholar] [CrossRef]
- Sainz, V.; Conniot, J.; de Matos, A.I.N.; Peres, C.; Zupanǒiǒ, E.; Moura, L.; Silva, L.; Florindo, H.F.; Gaspar, R.S. Regulatory aspects on nanomedicines. Biochem. Biophys. Res. Commun. 2015, 468, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; He, H.; Wu, Y.; Fan, J.; Cao, Y. Physiologically based pharmacokinetic modeling of nanoparticles. J. Pharm. Sci. 2018, 108, 58–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Human Medicines Development and Evaluation. In Proceedings of the 1st International Workshop on Nanomedicines 2010 Summary Report; European Medicines Agency: London, UK, 2010.
- Torqui, A.; Macau, A.M. Regional differences during the ICH regulatory consultation process between the EU, US, and Japan. Ther. Innov. Regul. Sci. 2018, 52, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Clogston, J.; Calzolai, L.; Rösslein, M.; Prina-Mello, A. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. J. Control. Release 2019, 299, 31–43. [Google Scholar] [CrossRef]
- Halamoda-Kenzaoui, B.; Box, H.; van Elk, M.; Gaitan, S.; Geertsma, R.E.; Lafuente, E.G.; Owen, A.; del Pozo, A.; Roesslein, M.; Bremer-Hoffmann, S. Launching stakeholder discussions on identified regulatory needs for nanotechnology-enabled health products. Precis. Nanomed. 2020, 3, 608–621. [Google Scholar] [CrossRef]
- Cicha, I.; Chauvierre, C.; Texier, I.; Cabella, C.; Metselaar, J.M.; Szebeni, J.; Dézsi, L.; Alexiou, C.; Rouzet, F.; Storm, G.; et al. From design to the clinic: Practical guidelines for translating cardiovascular nanomedicine. Cardiovasc. Res. 2018, 114, 1714–1727. [Google Scholar] [CrossRef] [Green Version]
- Van Gerven, J.; Cohen, A. Integrating data from the Investigational Medicinal Product Dossier/investigator’s brochure. A new tool for translational integration of preclinical effects. Br. J. Clin. Pharmacol. 2018, 84, 1457–1466. [Google Scholar] [CrossRef] [Green Version]
- European Committee for Human Medicinal Products. Reflection Paper on Nanotechnology-Based Medicinal Products for Human Use; European Medicines Agency Pre-Authorisation Evaluation of Medicines for Human Use: Amsterdam, The Netherlands, 2006.
- European Committee for Human Medicinal Products. Reflection Paper on the Data Requirements for Intravenous Liposomal Products Developed with Reference to An Innovator Liposomal Product; European Medicines Agency Pre-Authorisation Evaluation of Medicines for Human Use: Amsterdam, The Netherlands, 2013.
- European Committee for Human Medicinal Products. Reflection Paper on the Data Requirements for Intravenous Iron-Based Nano-Colloidal Products Developed with Reference to an Innovator Medicinal Product; European Medicines Agency Pre-Authorisation Evaluation of Medicines for Human Use: Amsterdam, The Netherlands, 2015.
- Credevo Trial Expert. The Drug Approval Process in Japan. Available online: https://credevo.com/articles/2020/04/15/the-drug-approval-process-in-japan/ (accessed on 30 December 2021).
- European Committee for Human Medicinal Products. Joint MHLW/EMA Reflection Paper on the Development of Block Copolymer Micelle Medicinal Products; European Medicines Agency Pre-Authorisation Evaluation of Medicines for Human Use: Amsterdam, The Netherlands, 2013.
- Japanese Ministry of Health, Labour and Welfare. Reflection Paper on Nucleic Acids (siRNA)-Loaded Nanotechnology-Based Drug Products; Japanese Ministry of Health, Labour and Welfare: Tokyo, Japan, 2016.
- De Vlieger, J.S.B.; Crommelin, D.J.A.; Tyner, K.; Drummond, D.C.; Jiang, W.; McNeil, S.E.; Neervannan, S.; Crist, R.; Shah, V.P. Report of the AAPS Guidance Forum on the FDA Draft Guidance for Industry: “Drug Products, Including Biological Products, that Contain Nanomaterials”. AAPS J. 2019, 21, 56. [Google Scholar] [CrossRef] [Green Version]
- Paradise, J. Regulating Nanomedicine at the Food and Drug Administration. AMA J. Ethic. 2019, 21, E347–E355. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration. Liposome Drug Products: Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation—Guidance for Industry; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Food and Drug Administration. Drug Products, Including Biological Products, that Contain Nanomaterials—Guidance for Industry; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2017.
- Cho, E.J.; Holback, H.; Liu, K.C.; Abouelmagd, S.A.; Park, J.; Yeo, Y. Nanoparticle characterization: State of the art, challenges, and emerging technologies. Mol. Pharm. 2013, 10, 2093–2110. [Google Scholar] [CrossRef] [Green Version]
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D.; et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part Fibre Toxicol. 2005, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Dukhin, S.S.; Labib, M.E. Convective diffusion of nanoparticles from the epithelial barrier toward regional lymph nodes. Adv. Colloid Interface Sci. 2013, 199-200, 23–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radomski, A.; Jurasz, P.; Alonso-Escolano, D.; Drews, M.; Morandi, M.; Malinski, T.; Radomski, M.W. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br. J. Pharmacol. 2005, 146, 882–893. [Google Scholar] [CrossRef] [Green Version]
- Madl, A.K.; Plummer, L.E.; Carosino, C.; Pinkerton, K.E. Nanoparticles, lung injury, and the role of oxidant stress. Annu. Rev. Physiol. 2014, 76, 447–465. [Google Scholar] [CrossRef] [Green Version]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Aravind, A.; Jeyamohan, P.; Nair, R.; Veeranarayanan, S.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. AS1411 aptamer tagged PLGA-lecithin-PEG nanoparticles for tumor cell targeting and drug delivery. Biotechnol. Bioeng. 2012, 109, 2920–2931. [Google Scholar] [CrossRef]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle surface functionalization: How to improve biocompatibility and cellular internalization. Front. Mol. Biosci. 2020, 7, 381. [Google Scholar] [CrossRef]
- Yue, L.; Sun, C.; Cheng, Q.; Ding, Y.; Wei, J.; Wang, R. Gold nanorods with a noncovalently tailorable surface for multi-modality image-guided chemo-photothermal cancer therapy. Chem. Commun. 2019, 55, 13506–13509. [Google Scholar] [CrossRef]
- Jung, E.; Kang, C.; Lee, J.; Yoo, D.; Hwang, D.W.; Kim, D.; Park, S.-C.; Lim, S.K.; Song, C.; Lee, D. Molecularly engineered theranostic nanoparticles for thrombosed vessels: H2O2-activatable contrast-enhanced photoacoustic imaging and antithrombotic therapy. ACS Nano 2017, 12, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Chen, T.; Ma, X.; Ren, W.; Zhou, Z.; Zhu, G.; Zhang, A.; Liu, Y.; Song, J.; Li, Z.; et al. Multifunctional theranostic nanoparticles based on exceedingly small magnetic iron oxide nanoparticles for T1-weighted magnetic resonance imaging and chemotherapy. ACS Nano 2017, 11, 10992–11004. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Florinas, S.; Teitgen, A.; Xu, Z.-Q.; Gao, C.; Wu, H.; Kataoka, K.; Cabral, H.; Christie, R.J. Controlled Fab installation onto polymeric micelle nanoparticles for tuned bioactivity. Sci. Technol. Adv. Mater. 2017, 18, 666–680. [Google Scholar] [CrossRef]
- Thiruppathi, R.; Mishra, S.; Ganapathy, M.; Padmanabhan, P.; Gulyás, B. Nanoparticle functionalization and its potentials for molecular imaging. Adv. Sci. 2016, 4, 1600279. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Fathian kolahkaj, F.; Derakhshandeh, K.; Khaleseh, F.; Azandaryani, A.H.; Mansouri, K.; Khazaei, M. Active targeting carrier for breast cancer treatment: Monoclonal antibody conjugated epirubicin loaded nanoparticle. J. Drug Deliv. Sci. Technol. 2019, 53, 101136. [Google Scholar] [CrossRef]
- Del Amo, L.; Cano, A.; Ettcheto, M.; Souto, E.; Espina, M.; Camins, A.; García, M.; Sánchez-López, E. Surface functionalization of PLGA nanoparticles to increase transport across the BBB for Alzheimer’s disease. Appl. Sci. 2021, 11, 4305. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, A.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z.; Sun, K. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 705–718. [Google Scholar] [CrossRef] [Green Version]
- Sanità, G.; Armanetti, P.; Silvestri, B.; Carrese, B.; Calì, G.; Pota, G.; Pezzella, A.; D’Ischia, M.; Luciani, G.; Menichetti, L.; et al. Albumin-modified melanin-silica hybrid nanoparticles target breast cancer cells via a SPARC-dependent mechanism. Front. Bioeng. Biotechnol. 2020, 8, 765. [Google Scholar] [CrossRef] [PubMed]
- Fornaguera, C.; Solans, C. Characterization of Polymeric Nanoparticle Dispersions for Biomedical Applications: Size, Surface Charge and Stability. Pharm. Nanotechnol. 2018, 6, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Okay, S. Single-molecule characterization of drug delivery systems. ASSAY Drug Dev. Technol. 2020, 18, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, U.M.; Marini, V.; Casiraghi, A.; Minghetti, P. Is the European regulatory framework sufficient to assure the safety of citizens using health products containing nanomaterials? Drug Discov. Today 2017, 22, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Menta, M.; Dacoba, T.G.; Crecente-Campo, J.; Alonso, M.J.; Dupin, D.; Loinaz, I.; Grassl, B.; Séby, F. Advanced nanomedicine characterization by DLS and AF4-UV-MALS: Application to a HIV nanovaccine. J. Pharm. Biomed. Anal. 2020, 179, 113017. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Takagi, M.; Murata, S.; Kato, M. Stability and drug release studies of an antimycotic nanomedicine using HPLC, dynamic light scattering and atomic force microscopy. J. Pharm Biomed. Anal. 2018, 148, 149–155. [Google Scholar] [CrossRef]
- Wren, S.; Minelli, C.; Pei, Y.; Akhtar, N. Evaluation of particle size techniques to support the development of manufacturing scale nanoparticles for application in pharmaceuticals. J. Pharm. Sci. 2020, 109, 2284–2293. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Caputo, F.; Arnould, A.; Bacia, M.; Ling, W.L.; Rustique, E.; Texier, I.; Mello, A.P.; Couffin, A.-C. Measuring particle size distribution by asymmetric flow field flow fractionation: A powerful method for the preclinical characterization of lipid-based nanoparticles. Mol. Pharm. 2019, 16, 756–767. [Google Scholar] [CrossRef] [Green Version]
- Mehn, D.; Caputo, F.; Rösslein, M.; Calzolai, L.; Saint-Antonin, F.; Courant, T.; Wick, P.; Gilliland, D. Larger or more? Nanoparticle characterisation methods for recognition of dimers. RSC Adv. 2017, 7, 27747–27754. [Google Scholar] [CrossRef] [Green Version]
- Varenne, F.; Makky, A.; Gaucher-Delmas, M.; Violleau, F.; Vauthier, C. Multimodal dispersion of nanoparticles: A comprehensive evaluation of size distribution with 9 size measurement methods. Pharm. Res. 2016, 33, 1220–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teulon, J.-M.; Godon, C.; Chantalat, L.; Moriscot, C.; Cambedouzou, J.; Odorico, M.; Ravaux, J.; Podor, R.; Gerdil, A.; Habert, A.; et al. On the operational aspects of measuring nanoparticle sizes. Nanomaterials 2018, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, M.; Fraschini, C.; Chauve, G.; Putaux, J.-L.; Moores, J.-L.P.A.A. Transmission Electron Microscopy for the Characterization of Cellulose Nanocrystals. In The Transmission Electron Microscope; Khan, M., Ed.; InTechOpen: London, UK, 2015; Volume 1, pp. 129–163. [Google Scholar]
- Rydz, J.; Šišková, A.; Eckstein, A.A. Scanning electron microscopy and atomic force microscopy: Topographic and dynamical surface studies of blends, composites, and hybrid functional materials for sustainable future. Adv. Mater. Sci. Eng. 2019, 2019, 6871785. [Google Scholar] [CrossRef] [Green Version]
- Su, D. Advanced electron microscopy characterization of nanomaterials for catalysis. Green Energy Environ. 2017, 2, 70–83. [Google Scholar] [CrossRef]
- Robson, A.-L.; Dastoor, P.C.; Flynn, J.; Palmer, W.; Martin, A.; Smith, D.W.; Woldu, A.; Hua, S. Advantages and limitations of current imaging techniques for characterizing liposome morphology. Front. Pharmacol. 2018, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Pujals, S.; Albertazzi, L. Super-resolution microscopy for nanomedicine research. ACS Nano 2019, 13, 9707–9712. [Google Scholar] [CrossRef]
- El-Say, K.M. Maximizing the encapsulation efficiency and the bioavailability of controlled-release cetirizine microspheres using Draper–Lin small composite design. Drug Des. Dev. Ther. 2016, 10, 825–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, S.; Pramanik, S.; Malgope, A. Formulation and optimization of sustained release stavudine microspheres using response surface methodology. Int. Scholarly Res. Notices 2011, 2011, 627623. [Google Scholar] [CrossRef] [Green Version]
- Gaikwad, V.L.; Choudhari, P.B.; Bhatia, N.M.; Bhatia, M.S. Chapter 2—Characterization of pharmaceutical nanocarriers: In vitro and in vivo studies. In Nanomaterials for Drug Delivery and Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 33–58. [Google Scholar]
- Daneshmand, S.; Golmohammadzadeh, S.; Jaafari, M.R.; Movaffagh, J.; Rezaee, M.; Sahebkar, A.; Malaekeh-Nikouei, B. Encapsulation challenges, the substantial issue in solid lipid nanoparticles characterization. J. Cell. Biochem. 2018, 119, 4251–4264. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Jahromi, L.P.; Ghazali, M.; Ashrafi, H.; Azadi, A. A comparison of models for the analysis of the kinetics of drug release from PLGA-based nanoparticles. Heliyon 2020, 6, e03451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojcik-Pastuszka, D.; Krzak, J.; Macikowski, B.; Berkowski, R.; Osiński, B.; Musiał, W. Evaluation of the release kinetics of a pharmacologically active substance from model intra-articular implants replacing the cruciate ligaments of the knee. Materials 2019, 12, 1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owonubi, S.J.; Aderibigbe, B.A.; Mukwevho, E.; Sadiku, R.; Ray, S.S. Characterization and in vitro release kinetics of antimalarials from whey protein-based hydrogel biocomposites. Int. J. Ind. Chem. 2018, 9, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Weng, J.; Tong, H.H.Y.; Chow, S.F. In Vitro release study of the polymeric drug nanoparticles: Development and validation of a novel method. Pharmaceutics 2020, 12, 732. [Google Scholar] [CrossRef]
- Shleghm, M.R.; Mircioiu, C.; Voicu, V.A.; Mircioiu, I.; Anuta, V. Estimation of the in vivo release of amiodarone from the pharmacokinetics of its active metabolite and correlation with its in vitro release. Front. Pharmacol. 2021, 11, 2383. [Google Scholar] [CrossRef]
- Maguire, C.M.; Rösslein, M.; Wick, P.; Prina-Mello, A. Characterisation of particles in solution—A perspective on light scattering and comparative technologies. Sci. Technol. Adv. Mater. 2018, 19, 732–745. [Google Scholar] [CrossRef]
- Kurakula, M.; Naveen, N.R. In situ gel loaded with chitosan-coated simvastatin nanoparticles: Promising delivery for effective anti-proliferative activity against tongue carcinoma. Mar. Drugs 2020, 18, 201. [Google Scholar] [CrossRef] [Green Version]
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Ryu, W.M.; Kim, S.-N.; Min, C.H.; Bin Choy, Y. Dry tablet formulation of PLGA nanoparticles with a preocular applicator for topical drug delivery to the eye. Pharmaceutics 2019, 11, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, G.; Bauer, M.; Haisch, C. Aerosol Generation from Laser-Ablation-Synthesized Nanoparticles; SPIE: Bellingham, WA, USA, 2020; Volume 11269. [Google Scholar]
- Feldman, D. Polymer nanocomposites in medicine. J. Macromol. Sci. Part A 2016, 53, 55–62. [Google Scholar] [CrossRef]
- Guterres, S.; Frank, L.A.; Sandri, G.; D’Autilia, F.; Contri, R.V.; Bonferoni, M.C.; Caramella, C.; Frank, A.G.; Pohlmann, A. Chitosan gel containing polymeric nanocapsules: A new formulation for vaginal drug delivery. Int. J. Nanomed. 2014, 9, 3151–3161. [Google Scholar] [CrossRef] [Green Version]
- Bremer-Hoffmann, S.; Halamoda-Kenzaoui, B.; Borgos, S.E. Identification of regulatory needs for nanomedicines. J. Interdiscip. Nanomed. 2018, 3, 4–15. [Google Scholar] [CrossRef]
- Gao, X.; Lowry, G.V. Progress towards standardized and validated characterizations for measuring physicochemical properties of manufactured nanomaterials relevant to nano health and safety risks. NanoImpact 2018, 9, 14–30. [Google Scholar] [CrossRef]
- Rodriguez-Garraus, A.; Azqueta, A.; Vettorazzi, A.; De Cerain, A.L. Genotoxicity of silver nanoparticles. Nanomaterials 2020, 10, 251. [Google Scholar] [CrossRef] [Green Version]
- Fornaguera, C.; Solans, C. Methods for the in vitro characterization of nanomedicines—Biological component interaction. J. Pers. Med. 2017, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization. Particle Size Analysis—Dynamic Light Scattering (DLS) (ISO 22412: 2017); International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- American Society for Testing and Materials. New Test Method Measuring the Size of Nanoparticles in Aqueous Media Using Batch-Mode Dynamic Light Scattering (ASTM WK54872); American Society for Testing and Materials: West Conshohocken, PA, USA, Under Development.
- Hackley, V.; Clogston, J. NIST-NCL Joint Assay Protocol, PCC-1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020. [CrossRef]
- International Organization for Standardization. Nanotechnologies—Measurements of Particle Size and Shape Distributions by Transmission Electron Microscopy (ISO 21363:2020); International Organization for Standardization: Geneva, Switzerland, 2020. [Google Scholar]
- International Organization for Standardization. Nanotechnologies—Measurements of Particle Size and Shape Distributions by Scanning Electron Microscopy (ISO 19749: 2021); International Organization for Standardization: Geneva, Switzerland, 2021. [Google Scholar]
- ASTM International. E56 Committee Guide for Size Measurement of Nanoparticles Using Atomic Force Microscopy; ASTM International: West Conshohocke, PA, USA, 2017. [Google Scholar] [CrossRef]
- ASTM International. E56 Committee Guide for Measurement of Particle Size Distribution of Nanomaterials in Suspension by Photon Correlation Spectroscopy (PCS); ASTM International: West Conshohocke, PA, USA, 2021. [Google Scholar] [CrossRef]
- Pease, L.; Tsai, D.-H.; Zangmeister, R.; Zachariah, M.; Tarlov, M. NIST-NCL Joint Assay Protocol, PCC-10; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020. [CrossRef]
- International Organization for Standardization. Determination of Particle Size Distribution by Centrifugal Liquid Sedimentation Methods—Part 2: Photocentrifuge Method (ISO 13318-2:2007); International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- International Organization for Standardization. Determination of Particle Size Distribution by Centrifugal Liquid Sedimentation Methods—Part 3: Centrifugal X-ray Method (ISO 13318-3: 2004); International Organization for Standardization: Geneva, Switzerland, 2004. [Google Scholar]
- ASTM International. E56 Committee Guide for Measurement of Particle Size Distribution of Nanomaterials in Suspension by Nanoparticle Tracking Analysis (NTA); ASTM International: West Conshohocke, PA, USA, 2018. [Google Scholar] [CrossRef]
- International Organization for Standardization. Determination of Particle Size Distribution—Electrical Sensing Zone Method—Part 1: Aperture/Orifice Tube Method (ISO 13319-1:2021); International Organization for Standardization: Geneva, Switzerland, 2021. [Google Scholar]
- International Organization for Standardization. Determination of Particle Size Distribution—Electrical Sensing Zone Method—Part 2: Tuneable Resistive Pulse Sensing Method (ISO / CD 13319-2); International Organization for Standardization: Geneva, Switzerland, Under development.
- Cleveland, D. NIST-NCL Joint Assay Protocol, PCC-14; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020. [CrossRef]
- International Organization for Standardization. Nanotechnologies—Characterization of Volatile Components in Single-Wall Carbon Nanotube Samples Using Evolved Gas Analysis/Gas Chromatograph-Mass Spectrometry (ISO/TS 11251:2019); International Organization for Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- International Organization for Standardization. Nanotechnologies—Determination of Elemental Impurities in Samples of Carbon Nanotubes Using Inductively Coupled Plasma Mass Spectrometry (ISO/TS 13278: 2017); International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Vermilya, A.; Clogston, J. NCL Method PCC-20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020. [CrossRef]
- Vermilya, A.; Clogston, J. NCL Method PCC-2; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020. [CrossRef]
- International Organization for Standardization. Guidelines for Good Practices in Zeta-Potential Measurement (ISO/TR 19997:2018); International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- International Organization for Standardization. Nanotechnologies—Analysis of Nano-Objects Using Asymmetrical-Flow and Centrifugal Field-Flow Fractionation (ISO/TS 21362: 2018); International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- Clogston, J.; Hu, Y. NCL Method PCC-19; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020. [CrossRef]
- International Organization for Standardization. Determination of the Specific Surface Area of Solids by Gas Adsorption—BET Method (ISO/DIS 9277); International Organization for Standardization: Geneva, Switzerland, Under development.
- International Organization for Standardization. Use of UV-Vis Absorption Spectroscopy in the Characterization of Cadmium Chalcogenide Colloidal Quantum Dots (ISO/TS 17466: 2015); International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- International Organization for Standardization. Particle Size Analysis—Particle Tracking Analysis (PTA) Method (ISO/WD 19430); International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- International Organization for Standardization. Nanotechnologies—Measurement Technique Matrix for the Characterization of Nano-objects (ISO/TR 18196:2016); International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- International Organization for Standardization. Nanotechnologies—Guidance on Physico-Chemical Characterization of Engineered Nanoscale Materials for Toxicologic Assessment—Technical Corrigendum 1 (ISO/TR 13014:2012/COR 1:2012); International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Chaudhary, K.; Masram, D.T. Biological Activities of Nanoparticles and Mechanism of Action. In Model Organisms to Study Biological Activities and Toxicity of Nanoparticles; Springer: Singapore, 2020; pp. 19–34. [Google Scholar] [CrossRef]
- Manzanares, D.; Ceña, V. Endocytosis: The nanoparticle and submicron nanocompounds gateway into the Cell. Pharmaceutics 2020, 12, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, P. Next-generation nanotech meds. EMBO Rep. 2016, 18, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonsson, M.K.B.; Van Veen, T.A.B.; Synnergren, J.; Becker, B. Towards creating the perfect in vitro cell model. Stem Cells Int. 2016, 2016, 3459730. [Google Scholar] [CrossRef] [Green Version]
- Cabrera-Pérez, M.; Sanz, M.B.; Sanjuan, V.M.; González-Álvarez, M.; Álvarez, I.G. Importance and applications of cell- and tissue-based in vitro models for drug permeability screening in early stages of drug development. In Concepts and Models for Drug Permeability Studies: Cell and Tissue Based In Vitro Culture Models; Sarmento, B., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 3–29. ISBN 9780081000946. [Google Scholar]
- Fröhlich, E. Comparison of conventional and advanced in vitro models in the toxicity testing of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1091–1107. [Google Scholar] [CrossRef] [Green Version]
- Rezaee, R.; Abdollahi, M. The importance of translatability in drug discovery. Expert Opin. Drug Discov. 2017, 12, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Alaylioglu, M.; Dursun, E.; Yilmazer, S.; Gezen-Ak, D. A Bridge between in vitro and in vivo studies in neuroscience: Organotypic brain slice cultures. Arch. Neuropsychiatry 2020, 57, 333–337. [Google Scholar] [CrossRef]
- Evans, S.J.; Clift, M.J.; Singh, N.; de Oliveira Mallia, J.; Burgum, M.; Wills, J.W.; Wilkinson, T.S.; Jenkins, G.J.; Doak, S.H. Critical review of the current and future challenges associated with advanced in vitro systems towards the study of nanoparticle (secondary) genotoxicity. Mutagenesis 2016, 32, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calienni, M.N.; Lillo, C.R.; Prieto, M.J.; Gorojod, R.M.; Alonso, S.D.V.; Kotler, M.L.; Gonzalez, M.; Montanari, J. Comparative toxicity of PEG and folate-derived blue-emitting silicon nanoparticles: In vitro and in vivo studies. Nanomedicine 2019, 14, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Fučíková, A.; Šmíd, B.; Nováková, J.; Matolínová, I.; Matolín, V.; Janata, M.; Bělinová, T.; Kalbáčová, M.H. Colloidal stability and catalytic activity of cerium oxide nanoparticles in cell culture media. RSC Adv. 2020, 10, 39373–39384. [Google Scholar] [CrossRef]
- Villanueva-Flores, F.; Castro-Lugo, A.; Ramírez, O.T.; Palomares, L.A. Understanding cellular interactions with nanomaterials: Towards a rational design of medical nanodevices. Nanotechnology 2020, 31, 132002. [Google Scholar] [CrossRef]
- Moore, T.L.; Urban, D.; Rodriguez-Lorenzo, L.; Milosevic, A.; Crippa, F.; Spuch-Calvar, M.; Balog, S.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle administration method in cell culture alters particle-cell interaction. Sci. Rep. 2019, 9, 900. [Google Scholar] [CrossRef]
- Berg, C.; Åberg, C. Quantitative analysis of nanoparticle transport through in vitro blood-brain barrier models. Tissue Barriers 2016, 4, e1143545. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, J.P.A.; Kim, B.Y.S.; Trounson, A. How to design preclinical studies in nanomedicine and cell therapy to maximize the prospects of clinical translation. Nat. Biomed. Eng. 2018, 2, 797–809. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Kloet, S.K.; Kezic, S.; Kuper, F.; Park, M.; Bellmann, S.; van der Zande, M.; Le Gac, S.; Krystek, P.; Peters, R.J.B.; et al. Progress and future of in vitro models to study translocation of nanoparticles. Arch. Toxicol. 2015, 89, 1469–1495. [Google Scholar] [CrossRef] [Green Version]
- Lazzari, S.; Moscatelli, D.; Codari, F.; Salmona, M.; Morbidelli, M.; Diomede, L. Colloidal stability of polymeric nanoparticles in biological fluids. J. Nanoparticle Res. 2012, 14, 920. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, C.; Schildknecht, S. In vitro research reproducibility: Keeping up high standards. Front. Pharmacol. 2019, 10, 1484. [Google Scholar] [CrossRef] [PubMed]
- Halamoda-Kenzaoui, B.; Holzwarth, U.; Roebben, G.; Bogni, A.; Bremer-Hoffmann, S. Mapping of the available standards against the regulatory needs for nanomedicines. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 11, e1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xavier, M.; García-Hevia, L.; Amado, I.R.; Pastrana, L.; Gonçalves, C. In vitro intestinal uptake and permeability of fluorescently-labelled hyaluronic acid nanogels. Int. J. Nanomed. 2019, 14, 9077–9088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Zhang, Y.S.; Zhang, X.; Liu, C. Organ-on-a-chip platforms for accelerating the evaluation of nanomedicine. Bioact. Mater. 2021, 6, 1012–1027. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J. Transwell® invasion assays. In Cell Migration; Methods in Molecular Biology (Methods and Protocols); Wells, C.M., Parsons, M., Eds.; Humana Press: New York, NY, USA, 2011; Volume 769, pp. 97–110. [Google Scholar]
- Coluccio, M.L.; Perozziello, G.; Malara, N.; Parrotta, E.; Zhang, P.; Gentile, F.; Limongi, T.; Raj, P.M.; Cuda, G.; Candeloro, P.; et al. Microfluidic platforms for cell cultures and investigations. Microelectron. Eng. 2019, 208, 14–28. [Google Scholar] [CrossRef]
- Rajasekaran, P.R.; Chapin, A.A.; Quan, D.N.; Herberholz, J.; Bentley, W.E.; Ghodssi, R. 3D-Printed electrochemical sensor-integrated transwell systems. Microsyst. Nanoeng. 2020, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Elberskirch, L.; Knoll, T.; Königsmark, R.; Renner, J.; Wilhelm, N.; von Briesen, H.; Wagner, S. Microfluidic 3D intestine tumor spheroid model for efficient in vitro investigation of nanoparticular formulations. J. Drug Deliv. Sci. Technol. 2021, 63, 102496. [Google Scholar] [CrossRef]
- Pellen-Mussi, P.; Tricot-Doleux, S.; Neaime, C.; Nerambourg, N.; Cabello-Hurtado, F.; Cordier, S.; Grasset, F.; Jeanne, S. Evaluation of functional SiO2 nanoparticles toxicity by a 3D culture model. J. Nanosci. Nanotechnol. 2018, 18, 3148–3157. [Google Scholar] [CrossRef]
- Fornaguera, C. Characterization of the interaction between nanomedicines and biological components: In vitro Evaluation. In Immune Aspects of Biopharmaceuticals and Nanomedicines; Bawa, R.S.J., Webster, T., Audette, G., Eds.; Jenny Stanford Publishing: New York, NY, USA, 2019; pp. 835–867. [Google Scholar]
- Malcolm, D.W.; Varghese, J.J.; Sorrells, J.E.; Ovitt, C.E.; Benoit, D.S.W. The effects of biological fluids on colloidal stability and siRNA delivery of a pH-responsive micellar nanoparticle delivery system. ACS Nano 2017, 12, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.M.; Poortinga, A.; Verhoeven, F.M.; Bonn, D.; Bonnet, S.; van Rijn, C.J.M. Degradation of lipid based drug delivery formulations during nebulization. Chem. Phys. 2021, 547, 111192. [Google Scholar] [CrossRef]
- Shaikh, M.V.; Kala, M.; Nivsarkar, M. Development and optimization of an ex vivo colloidal stability model for nanoformulations. AAPS PharmSciTech 2016, 18, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.K.; Simon, J.; Rosenauer, C.; Mailaender, V.; Morsbach, S.; Landfester, K. The transferability from animal models to humans: Challenges regarding aggregation and protein corona formation of nanoparticles. Biomacromolecules 2018, 19, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Bioanalytical Method Validation—Guidance for Industry; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Itabashi, D.; Murao, R.; Taniguchi, S.; Mizukami, K.; Takagi, H.; Kimura, M. Determination of size distribution of nanoparticles using asymmetric flow field-flow fractionation (AF4). ISIJ Int. 2020, 60, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid–polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Selby, L.I.; Johnston, A.P.R.; Such, G.K. The endosomal escape of nanoparticles: Toward more efficient cellular delivery. Bioconjugate Chem. 2018, 30, 263–272. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, S.M.; Rao, C.M.; Ahmad, M.F. Nanoparticle-Protein Interaction: The Significance and Role of Protein Corona. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2018; Volume 1048, pp. 175–198. ISBN 9783319720418. [Google Scholar]
- Moraru, C.; Mincea, M.; Menghiu, G.; Ostafe, V. Understanding the factors influencing chitosan-based nanoparticles-protein corona interaction and drug delivery applications. Molecules 2020, 25, 4758. [Google Scholar] [CrossRef]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent advances in understanding the protein corona of nanoparticles and in the formulation of “Stealthy” nanomaterials. Front. Bioeng. Biotechnol. 2020, 8, 166. [Google Scholar] [CrossRef]
- Sharifi, S.; Caracciolo, G.; Mahmoudi, M. Biomolecular Corona Affects Controlled Release of Drug Payloads from Nanocarriers. Trends Pharmacol. Sci. 2020, 41, 641–652. [Google Scholar] [CrossRef]
- Tirumala, M.G.; Anchi, P.; Raja, S.; Rachamalla, M.; Godugu, C. Novel methods and approaches for safety evaluation of nanoparticle formulations: A focus towards in vitro models and adverse outcome pathways. Front. Pharmacol. 2021, 12, 2157. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.P.; Ong, C.; Bay, B.H.; Baeg, G.H. Nanotoxicity: An interplay of oxidative stress, inflammation and cell death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, B.; Sunwoo, K.; Jangili, P.; Kim, J.; Kim, J.H.; Huang, M.; Xiong, J.; Sharma, A.; Yang, Z.; Qu, J.; et al. Nanomaterial designing strategies related to cell lysosome and their biomedical applications: A review. Biomaterials 2019, 211, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Lin, S.; Song, B.; Liu, J.; Lai, R.; Shao, L. The mechanisms of graphene-based materials-induced programmed cell death: A review of apoptosis, autophagy, and programmed necrosis. Int. J. Nanomed. 2017, 12, 6633–6646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boraschi, D.; Italiani, P.; Palomba, R.; Decuzzi, P.; Duschl, A.; Fadeel, B.; Moghimi, S.M. Nanoparticles and innate immunity: New perspectives on host defence. Semin. Immunol. 2017, 34, 33–51. [Google Scholar] [CrossRef]
- Casals, E.; Gonzalez, E.; Puntes, V.F. Reactivity of inorganic nanoparticles in biological environments: Insights into nanotoxicity mechanisms. J. Phys. D Appl. Phys. 2012, 45, 443001. [Google Scholar] [CrossRef] [Green Version]
- Albalawi, F.; Hussein, M.Z.; Fakurazi, S.; Masarudin, M.J. Engineered nanomaterials: The challenges and opportunities for nanomedicines. Int. J. Nanomed. 2021, 16, 161–184. [Google Scholar] [CrossRef]
- Labouta, H.I.; Asgarian, N.; Rinker, K.; Cramb, D.T. Meta-analysis of nanoparticle cytotoxicity via data-mining the literature. ACS Nano 2019, 13, 1583–1594. [Google Scholar] [CrossRef]
- Caster, J.M.; Yu, S.K.; Patel, A.N.; Newman, N.J.; Lee, Z.J.; Warner, S.; Wagner, K.T.; Roche, K.C.; Tian, X.; Min, Y.; et al. Effect of particle size on the biodistribution, toxicity, and efficacy of drug-loaded polymeric nanoparticles in chemoradiotherapy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1673–1683. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Adamska, A.; Bartoszewski, R.; Ochocka, J.R. Reuse of E-plate cell sensor arrays in the xCELLigence real-time cell analyzer. BioTechniques 2016, 61, 117–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Bando, K.; Mochizuki, K.; Taguchi, A.; Fujita, K.; Kawata, S. Quantitative evaluation of surface-enhanced raman scattering nanoparticles for intracellular pH sensing at a single particle level. Anal. Chem. 2019, 91, 3254–3262. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Yuan, C.; Feng, S.; Wang, L. Dark field digital holographic microscopy based on two-lens 360-degree oblique illumination. Curr. Opt. Photon. 2020, 4, 193–199. [Google Scholar]
- Kühn, J.; Shaffer, E.; Mena, J.; Breton, B.; Parent, J.; Rappaz, B.; Chambon, M.; Emery, Y.; Magistretti, P.J.; Depeursinge, C.; et al. Label-free cytotoxicity screening assay by digital holographic microscopy. ASSAY Drug Dev. Technol. 2013, 11, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Underwood, Z.E. A Genetic Approaches to Understand Peroxiredoxin-Mediated H2O2 Signalling Mechanisms. Ph.D Thesis, Newcastle University, Newcastle, UK, 2019. [Google Scholar]
- Zhang, Y.; Dai, M.; Yuan, Z. Methods for the detection of reactive oxygen species. Anal. Methods 2018, 10, 4625–4638. [Google Scholar] [CrossRef]
- Bouché, M.; Pühringer, M.; Iturmendi, A.; Amirshaghaghi, A.; Tsourkas, A.; Teasdale, I.; Cormode, D.P. Activatable hybrid polyphosphazene-AuNP nanoprobe for ROS detection by bimodal PA/CT imaging. ACS Appl. Mater. Interfaces 2019, 11, 28648–28656. [Google Scholar] [CrossRef] [PubMed]
- Erofeev, A.; Gorelkin, P.; Garanina, A.; Alova, A.; Efremova, M.; Vorobyeva, N.; Edwards, C.; Korchev, Y.; Majouga, A. Novel method for rapid toxicity screening of magnetic nanoparticles. Sci. Rep. 2018, 8, 7462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, B.C.; Wright, C.W.; Ibuki, Y.; Moreno-Villanueva, M.; Karlsson, H.L.; Hendriks, G.; Sims, C.; Singh, N.; Doak, S. Emerging metrology for high-throughput nanomaterial genotoxicology. Mutagenesis 2016, 32, 215–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendriks, G.; Derr, R.S.; Misovic, B.; Morolli, B.; Calléja, F.M.G.R.; Vrieling, H. The extended ToxTracker assay discriminates between induction of DNA damage, oxidative stress, and protein misfolding. Toxicol. Sci. 2015, 150, 190–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drasler, B.; Sayre, P.; Steinhäuser, K.G.; Petri-Fink, A.; Rothen-Rutishauser, B. In vitro approaches to assess the hazard of nanomaterials. NanoImpact 2017, 8, 99–116. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Wu, M.; Lv, J.; Yang, Y.; Dan, M.; Liu, L.; Chen, L.; Wu, X.; Fan, C.; et al. In vivo carcinogenicity study of silver nanoparticles in transgenic rasH2 mice by one single-dose intravenous administration. J. Nanoparticle Res. 2020, 22, 146. [Google Scholar] [CrossRef]
- Bressan, L.P.; Robles-Najar, J.; Adamo, C.B.; Quero, R.F.; Costa, B.M.C.; de Jesus, D.P.; da Silva, J.A.F. 3D-printed microfluidic device for the synthesis of silver and gold nanoparticles. Microchem. J. 2019, 146, 1083–1089. [Google Scholar] [CrossRef]
- Ma, L.; Wu, Y.; Li, Y.; Aazmi, A.; Zhou, H.; Zhang, B.; Yang, H. Current advances on 3D-bioprinted liver tissue models. Adv. Health Mater. 2020, 9, e2001517. [Google Scholar] [CrossRef]
- ICH Expert Working Group. S6 Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Brussels, Belgium, 1997. [Google Scholar]
- Organisation for Economic Co-Operation and Development. Guidance Document on Good In Vitro Method Practices (GIVIMP); Organisation for Economic Co-Operation and Development: Paris, France, 2018. [Google Scholar]
- European Committee for Human Medicinal Products. Reflection Paper Providing An Overview of the Current Regulatory Testing Requirements for Medicinal Products for Human Use and Opportunities for Implementation of the 3Rs; European Medicines Agency Pre-Authorisation Evaluation of Medicines for Human Use: Amsterdam, The Netherlands, 2018. [Google Scholar]
- ICH Expert Working Group. S8 Immunotoxicity Studies for Human Pharmaceuticals; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Brussels, Belgium, 2006. [Google Scholar]
- Hefti, F.F. Requirements for a lead compound to become a clinical candidate. BMC Neurosci. 2008, 9, S7. [Google Scholar] [CrossRef] [Green Version]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Blass, B.E. Basic Principles of Drug Discovery and Development; Academic Press: Cambridge, MA, USA, 2015; p. 582. [Google Scholar]
- Zwierzyna, M.; Overington, J.P. Classification and analysis of a large collection of in vivo bioassay descriptions. PLoS Comput. Biol. 2017, 13, e1005641. [Google Scholar] [CrossRef] [Green Version]
- Stern, S.; McNeil, S.; Patri, A.; Dobrovolskaia, M. Preclinical Characterization of Engineered Nanoparticles Intended for Cancer Therapeutics. In Nanotechnology for Cancer Therapy; CRC Press: Boca Raton, FL, USA, 2006; pp. 105–137. [Google Scholar]
- Moradi Kashkooli, F.; Soltani, M.; Souri, M.; Meaney, C.; Kohandel, M. Nexus between in silico and in vivo models to enhance clinical translation of nanomedicine. Nano Today 2021, 36, 101057. [Google Scholar] [CrossRef]
- Howard, J.; Murashov, V. Use of nanomaterials in animals. Appl. Biosaf. 2018, 23, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Tomalia, D.A.; Nixon, L.S.; Hedstrand, D.M. Engineering critical nanoscale design parameters (CNDPs): A strategy for developing effective nanomedicine therapies and assessing quantitative nanoscale structure-activity relationships (QNSARs). In Pharmaceutical Applications of Dendrimers; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–47. [Google Scholar] [CrossRef]
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alaraby, M.; Demir, E.; Domenech, J.; Velázquez, A.; Hernández, A.; Marcos, R. In vivo evaluation of the toxic and genotoxic effects of exposure to cobalt nanoparticles using Drosophila melanogaster. Environ. Sci. Nano 2019, 7, 610–622. [Google Scholar] [CrossRef]
- Johnston, L.J.; Gonzalez-Rojano, N.; Wilkinson, K.J.; Xing, B. Key challenges for evaluation of the safety of engineered nanomaterials. NanoImpact 2020, 18, 100219. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, S.; Kumar, V. Challenges for Assessing Toxicity of Nanomaterials. In Biochemical Toxicology—Heavy Metals and Nanomaterials; Ince, M., Kaplan, O., Ondrasek, G., Eds.; IntechOpen: London, UK, 2020; pp. 1–21. [Google Scholar]
- Dobrovolskaia, M.A. Pre-clinical immunotoxicity studies of nanotechnology-formulated drugs: Challenges, considerations and strategy. J. Control. Release 2015, 220, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Hannon, G.; Lysaght, J.; Liptrott, N.J.; Prina-Mello, A. Immunotoxicity considerations for next generation cancer nanomedicines. Adv. Sci. 2019, 6, 1900133. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.K.; Thareja, S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 524–539. [Google Scholar] [CrossRef] [Green Version]
- Desai, P.P.; Patravale, V.B. In Vitro–In Vivo Correlation for Pharmaceutical Nano-and Microsystems. In Characterization of Pharmaceutical Nano and Microsystems; Wiley: Hoboken, NJ, USA, 2021; pp. 137–170. [Google Scholar]
- Kumar, V.; Sharma, N.; Maitra, S.S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Szebeni, J.; Bedöcs, P.; Dezsi, L.; Urbanics, R. A porcine model of complement activation-related pseudoallergy to nano-pharmaceuticals: Pros and cons of translation to a preclinical safety test. Precis. Nanomed. 2018, 1, 63–73. [Google Scholar] [CrossRef]
- Käser, T. Swine as biomedical animal model for T-cell research—Success and potential for transmittable and non-transmittable human diseases. Mol. Immunol. 2021, 135, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, T.; Kozma, G.T.; Vashegyi, I.; Mészáros, T.; Rosivall, L.; Urbanics, R.; Storm, G.; Metselaar, J.M.; Szebeni, J. Liposome-induced hypersensitivity reactions: Risk reduction by design of safe infusion protocols in pigs. J. Control. Release 2019, 309, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Bawa, R. Human clinical relevance of the porcine model of pseudoallergic infusion reactions. Biomedicines 2020, 8, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baptista, C.V.J.; Faustino-Rocha, A.I.; Oliveira, P.A. Animal models in pharmacology: A brief history awarding the Nobel prizes for physiology or medicine. Pharmacology 2021, 106, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.; Obayashi, H.; Colandene, J.D.; Nesta, D.P. Japan-specific key regulatory aspects for development of new biopharmaceutical drug products. J. Pharm. Sci. 2018, 107, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Song, B.; Wu, J.; Zhang, Y.; Chen, A.; Shao, L. Potential adverse effects of nanoparticles on the reproductive system. Int. J. Nanomed. 2018, 13, 8487–8506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leite-Silva, V.R.; Lamer, M.L.; Sanchez, W.Y.; Liu, D.C.; Sanchez, W.H.; Morrow, I.; Martin, D.; Silva, H.D.T.; Prow, T.W.; Grice, J.E.; et al. The effect of formulation on the penetration of coated and uncoated zinc oxide nanoparticles into the viable epidermis of human skin in vivo. Eur. J. Pharm. Biopharm. 2013, 84, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Van Der Zande, M.; Vandebriel, R.J.; Groot, M.J.; Kramer, E.; Rivera, Z.E.H.; Rasmussen, K.; Ossenkoppele, J.S.; Tromp, P.; Gremmer, E.R.; Peters, R.J.; et al. Sub-chronic toxicity study in rats orally exposed to nanostructured silica. Part. Fibre Toxicol. 2014, 11, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valcourt, D.M.; Kapadia, C.H.; Scully, M.A.; Dang, M.N.; Day, E.S. Best practices for preclinical in vivo testing of cancer nanomedicines. Adv. Health Mater. 2020, 9, e2000110. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.Y.; DeLoid, G.M.; Pal, A.; Demokritou, P. Buoyant nanoparticles: Implications for nano-biointeractions in cellular studies. Small 2016, 12, 3172–3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laux, P.; Riebeling, C.; Booth, A.M.; Brain, J.D.; Brunner, J.; Cerrillo, C.; Creutzenberg, O.; Estrela-Lopis, I.; Gebel, T.; Johanson, G.; et al. Biokinetics of nanomaterials: The role of biopersistence. NanoImpact 2017, 6, 69–80. [Google Scholar] [CrossRef]
- Eskes, C.; Boström, A.-C.; Bowe, G.; Coecke, S.; Hartung, T.; Hendriks, G.; Pamies, D.; Piton, A.; Rovida, C. Good cell culture practices & in vitro toxicology. Toxicol. In Vitro 2017, 45, 272–277. [Google Scholar]
- Jankovic, S.; Kapo, B.; Sukalo, A.; Masic, I. Evaluation of published preclinical experimental studies in medicine: Methodology issues. Med. Arch. 2019, 73, 298–302. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Shurin, M.R.; Shvedova, A.A. Current understanding of interactions between nanoparticles and the immune system. Toxicol. Appl. Pharmacol. 2016, 299, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Cedrone, E.; Neun, B.W.; Rodriguez, J.; Vermilya, A.; Clogston, J.D.; McNeil, S.E.; Barenholz, Y.; Szebeni, J.; Dobrovolskaia, M.A. Anticoagulants influence the performance of in vitro assays intended for characterization of nanotechnology-based formulations. Molecules 2017, 23, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, M.; Björnmalm, M.; Thurecht, K.J.; Kent, S.J.; Parton, R.G.; Kavallaris, M.; Johnston, A.P.R.; Gooding, J.J.; Corrie, S.; Boyd, B.J.; et al. Minimum information reporting in bio–nano experimental literature. Nat. Nanotechnol. 2018, 13, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.W.; Wang, T.; Dixon, D. Toxicologic testing methods. In Loomis’s Essentials of Toxicology, 5th ed.; Hayes, A.W., Wang, T., Dixon, D., Eds.; Academic Press: Cambrigde, MA, USA, 2020; pp. 189–222. [Google Scholar]
- Dunnington, K.; Benrimoh, N.; Brandquist, C.; Cardillo-Marricco, N.; Di Spirito, M.; Grenier, J. Application of Pharmacokinetics in Early Drug Development. In Pharmacokinetics and Adverse Effects of Drugs-Mechanisms and Risks Factors; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Andrade, E.; Bento, A.; Cavalli, J.; Oliveira, S.; Schwanke, R.; Siqueira, J.; Freitas, C.; Marcon, R.; Calixto, J. Non-clinical studies in the process of new drug development—Part II: Good laboratory practice, metabolism, pharmacokinetics, safety and dose translation to clinical studies. Braz. J. Med. Biol. Res. 2016, 49, e5646. [Google Scholar] [CrossRef] [Green Version]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv. Transl. Res. 2021, 1–26. [Google Scholar] [CrossRef]
- Dawidczyk, C.M.; Russell, L.; Searson, P.C. Recommendations for benchmarking preclinical studies of nanomedicines. Cancer Res. 2015, 75, 4016–4020. [Google Scholar] [CrossRef] [Green Version]
- Aborig, M.; Malik, P.R.V.; Nambiar, S.; Chelle, P.; Darko, J.; Mutsaers, A.; Edginton, A.N.; Fleck, A.; Osei, E.; Wettig, S. Biodistribution and physiologically-based pharmacokinetic modeling of gold nanoparticles in mice with interspecies extrapolation. Pharmaceutics 2019, 11, 179. [Google Scholar] [CrossRef] [Green Version]
- Freitag, T.L.; Podojil, J.R.; Pearson, R.M.; Fokta, F.J.; Sahl, C.; Messing, M.; Andersson, L.C.; Leskinen, K.; Saavalainen, P.; Hoover, L.I.; et al. Gliadin nanoparticles induce immune tolerance to gliadin in mouse models of Celiac disease. Gastroenterology 2020, 158, 1667–1681.e12. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, W.C.; Szebeni, J.; Kozlov, S.V.; Lucas, A.T.; Piscitelli, J.A.; Dobrovolskaia, M.A. Animal models for analysis of immunological responses to nanomaterials: Challenges and considerations. Adv. Drug Deliv. Rev. 2018, 136–137, 82–96. [Google Scholar] [CrossRef]
- Szebeni, J. Hemocompatibility testing for nanomedicines and biologicals: Predictive assays for complement mediated infusion reactions. Eur. J. Nanomed. 2012, 4, 33–53. [Google Scholar] [CrossRef]
- ICH Expert Working Group. Pharmaceutical Development Q8(R2); International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Brussels, Belgium, 2009. [Google Scholar]
- Aguilar-Pérez, K.M.; Avilés-Castrillo, J.I.; Medina, D.I.; Parra-Saldivar, R.; Iqbal, H.M.N. Insight into nanoliposomes as smart nanocarriers for greening the twenty-first century biomedical settings. Front. Bioeng. Biotechnol. 2020, 8, 1441. [Google Scholar] [CrossRef]
- Zolnik, B.S.; Sadrieh, N. Regulatory perspective on the importance of ADME assessment of nanoscale material containing drugs. Adv. Drug Deliv. Rev. 2009, 61, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, S.; Suthar, J.; Rokade, R.; Deshpande, P.; Singh, P.; Pratinidhi, A.; Khambadkhar, R.; Utekar, S. Pharmacokinetics, metabolism, distribution and permeability of nanomedicine. Curr. Drug Metab. 2018, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.B.; Dobrovolskaia, M.A.; Patri, A.K.; McNeil, S.E. Characterization of nanoparticles for therapeutics. Nanomedicine 2007, 2, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.T.; Martinez, M.N.; Stevens, D.M. When is it important to measure unbound drug in evaluating nanomedicine pharmacokinetics? Drug Metab. Dispos. 2016, 44, 1934–1939. [Google Scholar] [CrossRef] [Green Version]
- Noda Albelo, A.; Vidal Tallet, A. Farmacocinética y farmacodinámica, implicación en un uso más racional de los antimicrobianos en pediatría. Rev. Médica Electrónica 2009, 31. [Google Scholar]
- Feitosa, R.D.C.; Geraldes, D.C.; Beraldo-De-Araújo, V.; Costa, J.S.R.; Oliveira-Nascimento, L. Pharmacokinetic aspects of nanoparticle-in-Matrix drug delivery systems for oral/buccal delivery. Front. Pharmacol. 2019, 10, 1057. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.H.; Han, H.-K. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018, 48, 43–60, Correction in J. Pharm. Investig. 2018, 49, 201. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, Y.; Higashisaka, K.; Tsunoda, S.-I.; Tsutsumi, Y. The Absorption, Distribution, Metabolism, and Excretion Profile of Nanoparticles. In Engineered Cell Manipulation for Biomedical Application; Akashi, M., Akagi, T., Matsusaki, M., Eds.; Springer: Tokyo, Japan, 2014; pp. 259–271. [Google Scholar]
- Xu, Y.; Zheng, Y.; Wu, L.; Zhu, X.; Zhang, Z.; Huang, Y. Novel solid lipid nanoparticle with endosomal escape function for oral delivery of insulin. ACS Appl. Mater. Interfaces 2018, 10, 9315–9324. [Google Scholar] [CrossRef]
- Raza, K.; Kumar, P.; Kumar, N.; Malik, R. Pharmacokinetics and biodistribution of the nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Nimesh, S., Chandra, R., Gupta, N., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 165–186. [Google Scholar]
- Chowdhury, E.H. Pharmacokinetics and biodistribution of nanoparticles. In Nanotherapeutics; Chowdhury, E.H., Ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Inoue, M.; Sakamoto, K.; Suzuki, A.; Nakai, S.; Ando, A.; Shiraki, Y.; Nakahara, Y.; Omura, M.; Enomoto, A.; Nakase, I.; et al. Size and surface modification of silica nanoparticles affect the severity of lung toxicity by modulating endosomal ROS generation in macrophages. Part. Fibre Toxicol. 2021, 18, 21. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Liu, X.; Kumar, S.; Gochman, G.; Ji, Y.; Liao, Y.-P.; Chang, C.H.; Situ, W.; Lu, J.; et al. Use of polymeric nanoparticle platform targeting the liver to induce treg-mediated antigen-specific immune tolerance in a pulmonary allergen sensitization model. ACS Nano 2019, 13, 4778–4794. [Google Scholar] [CrossRef]
- Bartlett, J.A.; Brewster, M.; Brown, P.; Cabral-Lilly, D.; Cruz, C.N.; David, R.; Eickhoff, W.M.; Haubenreisser, S.; Jacobs, A.; Malinoski, F.; et al. Summary report of PQRI workshop on nanomaterial in drug products: Current experience and management of potential risks. AAPS J. 2014, 17, 44–64. [Google Scholar] [CrossRef]
- Tavakol, M.; Montazeri, A.; Naghdabadi, R.; Hajipour, M.J.; Zanganeh, S.; Caracciolo, G.; Mahmoudi, M. Disease-related metabolites affect protein–nanoparticle interactions. Nanoscale 2018, 10, 7108–7115. [Google Scholar] [CrossRef]
- Webborn, P.J. The role of pharmacokinetic studies in drug discovery: Where are we now, how did we get here and where are we going? Futur. Med. Chem. 2014, 6, 1233–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.-J.; Zhang, J. Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics 2020, 10, 4944–4957. [Google Scholar] [CrossRef]
- Li, M.; Zou, P.; Tyner, K.; Lee, S. Physiologically based pharmacokinetic (PBPK) modeling of pharmaceutical nanoparticles. AAPS J. 2016, 19, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Valic, M.S.; Zheng, G. Research tools for extrapolating the disposition and pharmacokinetics of nanomaterials from preclinical animals to humans. Theranostics 2019, 9, 3365–3387. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Quan, L.; Zhou, C.; Zhan, Q. Factors relating to the biodistribution & clearance of nanoparticles & their effects on in vivo application. Nanomedicine 2018, 13, 1495–1512. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Italiani, P. Model Validity in nanoimmunosafety: Advantages and Disadvantages of in vivo vs in vitro models, and human vs. animal models. Curr. Bionanotechnol. 2017, 2, 71–76. [Google Scholar] [CrossRef]
- Bernal-Chávez, S.; Del Prado-Audelo, M.; Caballero-Florán, I.; Giraldo-Gomez, D.; Figueroa-Gonzalez, G.; Reyes-Hernandez, O.; Carmen, M.G.-D.; González-Torres, M.; Cortés, H.; Leyva-Gómez, G. Insights into terminal sterilization processes of nanoparticles for biomedical applications. Molecules 2021, 26, 2068. [Google Scholar] [CrossRef] [PubMed]
- Mangini, M.; Verde, A.; Boraschi, D.; Puntes, V.F.; Italiani, P.; De Luca, A.C. Interaction of nanoparticles with endotoxin Importance in nanosafety testing and exploitation for endotoxin binding. Nanotoxicology 2021, 15, 558–576. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, Z.; Radauer-Preiml, I.; Andosch, A.; Casals, E.; Luetz-Meindl, U.; Cobaleda, M.; Lin, Z.; Jaberi-Douraki, M.; Italiani, P.; et al. Bacterial endotoxin (lipopolysaccharide) binds to the surface of gold nanoparticles, interferes with biocorona formation and induces human monocyte inflammatory activation. Nanotoxicology 2017, 11, 1157–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetten, M.A.; Gulumian, M. Interference of Gold Nanoparticles with In vitro Endotoxin Detection Assays. Curr. Nanosci. 2020, 16, 204–213. [Google Scholar] [CrossRef]
- Zielińska, A.; Soles, B.B.; Lopes, A.R.; Vaz, B.F.; Rodrigues, C.M.; Alves, T.F.R.; Klensporf-Pawlik, D.; Durazzo, A.; Lucarini, M.; Severino, P.; et al. Nanopharmaceuticals for eye administration: Sterilization, depyrogenation and clinical applications. Biology 2020, 9, 336. [Google Scholar] [CrossRef]
- Li, Y.; Fujita, M.; Boraschi, D. Endotoxin contamination in nanomaterials leads to the misinterpretation of immunosafety results. Front. Immunol. 2017, 8, 472. [Google Scholar] [CrossRef]
- Engin, A.B.; Hayes, A.W. The impact of immunotoxicity in evaluation of the nanomaterials safety. Toxicol. Res. Appl. 2018, 2, 2397847318755579. [Google Scholar] [CrossRef] [Green Version]
- Ahamad, N.; Bhardwaj, P.; Bhatia, E.; Banerjee, R. Clinical toxicity of nanomedicines. In Nano Medicine and Nano Safety; Springer: Singapore, 2020; pp. 533–560. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Elzeny, H.; Zhang, F.; Ali, E.N.; Fathi, H.A.; Zhang, S.; Li, R.; El-Mokhtar, M.A.; Hamad, M.A.; Wooley, K.L.; Elsabahy, M. Polyphosphoester nanoparticles as biodegradable platform for delivery of multiple drugs and siRNA. Drug Des. Dev. Ther. 2017, 11, 483–496. [Google Scholar] [CrossRef]
- Forest, V.; Hochepied, J.-F.; Pourchez, J. Importance of choosing relevant biological end points to predict nanoparticle toxicity with computational approaches for human health risk assessment. Chem. Res. Toxicol. 2019, 32, 1320–1326. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, properties, and regulatory issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Bawa, R.; Audette, G.F.; Reese, B. Handbook of Clinical Nanomedicine: Law, Business, Regulation, Safety, and Risk; Jenny Stanford Publishing: New York, NY, USA, 2016. [Google Scholar]
- ICH Expert Working Group. M3(R2) Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Brussels, Belgium, 2010. [Google Scholar]
- ICH Expert Working Group. S9 Nonclinical Evaluation for Anticancer Pharmaceuticals; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Brussels, Belgium, 2008. [Google Scholar]
- ICH Expert Working Group. S4 Duration of Chronic Toxicity Testing in Animals (Rodent and Non Rodent Toxicity Testing); International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Brussels, Belgium, 1999. [Google Scholar]
- Arts, J.H.E.; Irfan, M.-A.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Neubauer, N.; Petry, T.; Sauer, U.G.; et al. Case studies putting the decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping) into practice. Regul. Toxicol. Pharmacol. 2016, 76, 234–261. [Google Scholar] [CrossRef] [Green Version]
- Rycroft, T.; Trump, B.; Poinsatte-Jones, K.; Linkov, I. Nanotoxicology and nanomedicine: Making development decisions in an evolving governance environment. J. Nanoparticle Res. 2018, 20, 52. [Google Scholar] [CrossRef]
- ICH Expert Working Group. S3B Pharmacokinetics: Repeated Dose Tissue Distribution Studies; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Brussels, Belgium, 1995. [Google Scholar]
- Nanotechnology Characterization Laboratory. Pharmacology and Toxicology Characterization of Nanomedicines; Nanotechnology Characterization Laboratory: Frederick, MD, USA, 2020.
- Sahibzada, M.U.K.; Zahoor, M.; Sadiq, A.; ur Rehman, F.; Al-Mohaimeed, A.M.; Shahid, M.; Naz, S.; Ullah, R. Bioavailability and hepatoprotection enhancement of berberine and its nanoparticles prepared by liquid antisolvent method. Saudi J. Biol. Sci. 2021, 28, 327–332. [Google Scholar] [CrossRef]
- Skoczen, S.L.; Snapp, K.S.; Crist, R.; Kozak, D.; Jiang, X.; Liu, H.; Stern, S.T. Distinguishing pharmacokinetics of marketed nanomedicine formulations using a stable isotope tracer assay. ACS Pharmacol. Transl. Sci. 2020, 3, 547–558. [Google Scholar] [CrossRef] [Green Version]
- Pita, R. The EU Regulatory Landscape of Non-Biological Complex Drugs. In Non-Biological Complex Drugs; AAPS Advances in the Pharmaceutical Sciences Series; Crommelin, D., De Vlieger, J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 20, pp. 357–380. [Google Scholar]
- Goto, A.; Abe, S.; Koshiba, S.; Yamaguchi, K.; Sato, N.; Kurahashi, Y. Current status and future perspective on preclinical pharmacokinetic and pharmacodynamic (PK/PD) analysis: Survey in Japan pharmaceutical manufacturers association (JPMA). Drug Metab. Pharmacokinet. 2019, 34, 148–154. [Google Scholar] [CrossRef]
- Etuntland, T.; Eethell, B.; Ekosaka, T.; Eblasco, F.; Zang, R.X.; Ejain, M.; Egould, T.; Ehoffmaster, K. Implementation of pharmacokinetic and pharmacodynamic strategies in early research phases of drug discovery and development at Novartis Institute of Biomedical Research. Front. Pharmacol. 2014, 5, 174. [Google Scholar] [CrossRef] [Green Version]
- Giannakou, C.; Park, M.V.; De Jong, W.H.; Van Loveren, H.; Vandebriel, R.J.; Geertsma, R. A comparison of immunotoxic effects of nanomedicinal products with regulatory immunotoxicity testing requirements. Int. J. Nanomed. 2016, 11, 2935–2952. [Google Scholar] [CrossRef] [Green Version]
- Burleson, S.C.M.; Freebern, W.J.; Burleson, F.G.; Burleson, G.R.; Johnson, V.J.; Luebke, R.W. Host Resistance Assays; Springer: New York, NY, USA, 2018; pp. 117–145. [Google Scholar]
- EFSA Scientific Committee; Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; et al. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16, e05327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannakou, C.; Park, M.V.D.Z.; Bosselaers, I.E.M.; de Jong, W.H.; van der Laan, J.W.; van Loveren, H.; Vandebriel, R.J.; Geertsma, R.E. Nonclinical regulatory immunotoxicity testing of nanomedicinal products: Proposed strategy and possible pitfalls. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Felice, G.; Colombo, P. Nanoparticle–allergen complexes for allergen immunotherapy. Int. J. Nanomed. 2017, 12, 4493–4504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanotechnology Characterization Laboratory. Assay Cascade Protocols. Available online: https://ncl.cancer.gov/resources/assay-cascade-protocols (accessed on 3 October 2021).
- Hannon, G.; Prina-Mello, A. Endotoxin contamination of engineered nanomaterials: Overcoming the hurdles associated with endotoxin testing. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1738. [Google Scholar] [CrossRef]
- Steinová, J.; Bobčíková, K.; Hristov, D.R.; Ševců, A. Evaluation of two different methods for endotoxin detection in nanoparticle suspensions. In Proceedings of the Nanocon 2016, Brno, Czech Republic, 19–21 October 2016. [Google Scholar]
- Himly, M.; Geppert, M.; Hofer, S.; Hofstaetter, N.; Horejs-Höck, J.; Duschl, A. When would immunologists consider a nanomaterial to be safe? Recommendations for planning studies on nanosafety. Small 2020, 16, e1907483. [Google Scholar] [CrossRef] [Green Version]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2021. [Google Scholar]
- Subbarao, N. Nanoparticle Sterility and Sterilization of Nanomaterials. In Handbook of Immunological Properties of Engineered Nanomaterials; Dobrovolskaia, M., McNeil, S., Eds.; World Scientific: Singapore, 2016; pp. 53–75. [Google Scholar]
- Halamoda-Kenzaoui, B.; Baconnier, S.; Bastogne, T.; Bazile, D.; Boisseau, P.; Borchard, G.; Borgos, S.E.; Calzolai, L.; Cederbrant, K.; Di Felice, G.; et al. Bridging communities in the field of nanomedicine. Regul. Toxicol. Pharmacol. 2019, 106, 187–196. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Mansoori, G.A. Large-scale production/biosynthesis of biogenic nanoparticles. In Biogenic Nano-Particles and Their Use in Agro-Ecosystems; Springer: Singapore, 2020; pp. 67–83. [Google Scholar] [CrossRef]
- Feng, J.; Markwalter, C.E.; Tian, C.; Armstrong, M.; Prud’Homme, R.K. Translational formulation of nanoparticle therapeutics from laboratory discovery to clinical scale. J. Transl. Med. 2019, 17, 200. [Google Scholar] [CrossRef]
- Emily, M.; Ioanna, N.; Scott, B.; Beat, F. Reflections on FDA draft guidance for products containing nanomaterials: Is the abbreviated new drug application (ANDA) a suitable pathway for nanomedicines? AAPS J. 2018, 20, 92. [Google Scholar] [CrossRef] [Green Version]
- Gdowski, A.; Johnson, K.; Shah, S.; Gryczynski, I.; Vishwanatha, J.; Ranjan, A. Optimization and scale up of microfluidic nanolipomer production method for preclinical and potential clinical trials. J. Nanobiotechnol. 2018, 16, 12. [Google Scholar] [CrossRef] [Green Version]
- Aerosol Processes. In Design and Processing of Particulate Products; Cambridge Series in Chemical Engineering; Litster, J. (Ed.) Cambridge University Press: Cambridge, UK, 2016; pp. 161–191. [Google Scholar]
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.-F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, N.K.; Kaushik, N.; Linh, N.N.; Ghimire, B.; Pengkit, A.; Sornsakdanuphap, J.; Lee, S.-J.; Choi, E.H. Plasma and nanomaterials: Fabrication and biomedical applications. Nanomaterials 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piszczek, P.; Radtke, A. Silver nanoparticles fabricated using chemical vapor deposition and atomic layer deposition techniques: Properties, applications and perspectives: Review. In Noble and Precious Metals; Seehra, M.S., Bristow, A.D., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, G.V. Introductory Chapter: A Brief Semblance of the Sol-Gel Method in Research. In Sol-Gel Method-Design and Synthesis of New Materials with Interesting Physical, Chemical and Biological Properties; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- DeFrates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein polymer-based nanoparticles: Fabrication and medical applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: Synthesis, characterization and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parhizkar, M.; Mahalingam, S.; Homer-Vanniasinkam, S.; Edirisinghe, M. Latest developments in innovative manufacturing to combine nanotechnology with healthcare. Nanomedicine 2018, 13, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.A.; Costales, A.B.; Jaffari, M.; Urbauer, D.L.; Frumovitz, M.; Kutac, C.K.; Tran, H.; Coleman, R.L. Is it equivalent? Evaluation of the clinical activity of single agent Lipodox® compared to single agent Doxil® in ovarian cancer treatment. J. Oncol. Pharm. Pr. 2015, 22, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Rivnay, B.; Wakim, J.; Avery, K.; Petrochenko, P.; Myung, J.H.; Kozak, D.; Yoon, S.; Landrau, N.; Nivorozhkin, A. Critical process parameters in manufacturing of liposomal formulations of amphotericin B. Int. J. Pharm. 2019, 565, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Roces, C.B.; Port, E.C.; Daskalakis, N.N.; Watts, J.A.; Aylott, J.W.; Halbert, G.W.; Perrie, Y. Rapid scale-up and production of active-loaded PEGylated liposomes. Int. J. Pharm. 2020, 586, 119566. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.M.; Nguyen, D.X.; Patel, P.; Cote, B.; Al-Fatease, A.; Pham, Y.; Huynh, M.G.; Woo, Y.; Alani, A.W. Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Koynova, R.T.B. Recent Progress in Liposome Production, Relevance to Drug Delivery and Nanomedicine. Recent Pat. Nanotechnol. 2015, 9, 86–93. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef]
- Zare, Y. Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties. Compos. Part A Appl. Sci. Manuf. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef]
- Phan, H.; Haes, A.J. What does nanoparticle stability mean? J. Phys. Chem. C 2019, 123, 16495–16507. [Google Scholar] [CrossRef]
- Raval, N.; Jogi, H.; Gondaliya, P.; Kalia, K.; Tekade, R.K. Method and its composition for encapsulation, stabilization, and delivery of siRNA in anionic polymeric nanoplex: An in vitro-in vivo assessment. Sci. Rep. 2019, 9, 16047. [Google Scholar] [CrossRef] [Green Version]
- Giovannini, G.; Warncke, P.; Fischer, D.; Stranik, O.; Hall, A.; Gubala, V. Improving colloidal stability of silica nanoparticles when stored in responsive gel: Application and toxicity study. Nanotoxicology 2018, 12, 407–422. [Google Scholar] [CrossRef]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine scale-up technologies: Feasibilities and challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Ribes, A.; Sánchez-Cabezas, S.; Hernández-Montoto, A.; Villaescusa, L.A.; Aznar, E.; Martínez-Máñez, R.; Marcos, M.D.; López-Tendero, M.J.; Pradas, S.; Cuenca-Bustos, A. Lab and pilot-scale synthesis of MxOm@SiC core–shell nanoparticles. Materials 2020, 13, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Ashton, S.; Song, Y.H.; Nolan, J.; Cadogan, E.; Murray, J.; Odedra, R.; Foster, J.; Hall, P.A.; Low, S.; Taylor, P.; et al. Aurora kinase inhibitor nanoparticles target tumors with favorable therapeutic index in vivo. Sci. Transl. Med. 2016, 8, 325ra17. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, X.; Le, Y. Amorphous nanoparticulate formulation of sirolimus and its tablets. Pharmaceutics 2018, 10, 155. [Google Scholar] [CrossRef] [Green Version]
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, C.G.; Tagit, O. PLGA-based nanomedicines manufacturing: Technologies overview and challenges in industrial scale-up. Int. J. Pharm. 2021, 605, 120807. [Google Scholar] [CrossRef]
- Peltonen, L. Practical guidelines for the characterization and quality control of pure drug nanoparticles and nano-cocrystals in the pharmaceutical industry. Adv. Drug Deliv. Rev. 2018, 131, 101–115. [Google Scholar] [CrossRef]
- Oktay, A.N.; Ilbasmis-Tamer, S.; Celebi, N. The effect of critical process parameters of the high pressure homogenization technique on the critical quality attributes of flurbiprofen nanosuspensions. Pharm. Dev. Technol. 2019, 24, 1278–1286. [Google Scholar] [CrossRef]
- Operti, M.C.; Fecher, D.; van Dinther, E.A.W.; Grimm, S.; Jaber, R.; Figdor, C.G.; Tagit, O. A comparative assessment of continuous production techniques to generate sub-micron size PLGA particles. Int. J. Pharm. 2018, 550, 140–148. [Google Scholar] [CrossRef]
- He, H.; Liu, L.; Morin, E.E.; Liu, M.; Schwendeman, A. Survey of clinical translation of cancer nanomedicines—Lessons learned from successes and failures. Acc. Chem. Res. 2019, 52, 2445–2461. [Google Scholar] [CrossRef]
- Rawal, M.; Singh, A.; Amiji, M.M. Quality-by-design concepts to improve nanotechnology-based drug development. Pharm. Res. 2019, 36, 153. [Google Scholar] [CrossRef] [PubMed]
- ICH Expert Working Group. Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients—Guidance for Industry; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Brussels, Belgium, 2016. [Google Scholar]
- ICH Expert Working Group. Q9 on Quality Risk Management; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Brussels, Belgium, 2015. [Google Scholar]
- ICH Expert Working Group. Q10 on Pharmaceutical Quality System; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Brussels, Belgium, 2015. [Google Scholar]
- ICH Expert Working Group. Q11 on Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities); International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Brussels, Belgium, 2011. [Google Scholar]
- Politis, S.N.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of experiments (DoE) in pharmaceutical development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef]
- Soni, G.; Kale, K.; Shetty, S.; Gupta, M.; Yadav, K.S. Quality by design (QbD) approach in processing polymeric nanoparticles loading anticancer drugs by high pressure homogenizer. Heliyon 2020, 6, e03846. [Google Scholar] [CrossRef] [PubMed]
- Alt, N.; Zhang, T.Y.; Motchnik, P.; Taticek, R.; Quarmby, V.; Schlothauer, T.; Beck, H.; Emrich, T.; Harris, R.J. Determination of critical quality attributes for monoclonal antibodies using quality by design principles. Biologicals 2016, 44, 291–305. [Google Scholar] [CrossRef]
- Cunha, S.; Costa, C.P.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Using the quality by design (QbD) approach to optimize formulations of lipid nanoparticles and nanoemulsions: A review. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102206. [Google Scholar] [CrossRef] [PubMed]
- Hejmady, S.; Singhvi, G.; Saha, R.N.; Dubey, S.K. Regulatory aspects in process development and scale-up of nanopharmaceuticals. Ther. Deliv. 2020, 11, 341–343. [Google Scholar] [CrossRef]
- Troiano, G.; Nolan, J.; Parsons, D.; Van Geen Hoven, C.; Zale, S. A Quality by Design Approach to Developing and Manufacturing Polymeric Nanoparticle Drug Products. AAPS J. 2016, 18, 1354–1365. [Google Scholar] [CrossRef]
- Dormont, F.; Rouquette, M.; Mahatsekake, C.; Gobeaux, F.; Peramo, A.; Brusini, R.; Calet, S.; Testard, F.; Lepetre-Mouelhi, S.; Desmaële, D.; et al. Translation of nanomedicines from lab to industrial scale synthesis: The case of squalene-adenosine nanoparticles. J. Control. Release 2019, 307, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiao, Y.; Wu, Z. Nanosystem trends in drug delivery using quality-by-design concept. J. Control. Release 2017, 256, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Flühmann, B.; Prithviraj, R. Nanomedicines & Nanosimilars: Implications for Regulators, Payers, and Prescribers. In Proceedings of the Summary of Key Discussion Points from DIA Europe, Viena, Austria, 5–7 February 2019. [Google Scholar]
- Beg, S.; Saini, S.; Bandopadhyay, S.; Katare, O.P.; Singh, B. QbD-driven development and evaluation of nanostructured lipid carriers (NLCs) of Olmesartan medoxomil employing multivariate statistical techniques. Drug Dev. Ind. Pharm. 2017, 44, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.M.; Shelat, P.K.; Lalwani, A.N. QbD based development of proliposome of lopinavir for improved oral bioavailability. Eur. J. Pharm. Sci. 2017, 108, 50–61. [Google Scholar] [CrossRef]
- Leng, D.; Thanki, K.; Fattal, E.; Foged, C.; Yang, M. Engineering of budesonide-loaded lipid-polymer hybrid nanoparticles using a quality-by-design approach. Int. J. Pharm. 2018, 548, 740–746. [Google Scholar] [CrossRef]
- Hahn, S.M. Accelerating the Adoption of Advanced Manufacturing Technologies to Strengthen Our Public Health Infrastructure. Available online: https://www.fda.gov/news-events/fda-voices/accelerating-adoption-advanced-manufacturing-technologies-strengthen-our-public-health (accessed on 3 October 2021).
- Accomasso, L.; Cristallini, C.; Giachino, C. Risk Assessment and Risk Minimization in Nanomedicine: A Need for Predictive, Alternative, and 3Rs Strategies. Front. Pharmacol. 2018, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Barré-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Futur. Sci. OA 2015, 1, FSO63. [Google Scholar] [CrossRef] [Green Version]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. New Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Holbein, M.E.B. Understanding FDA regulatory requirements for investigational new drug applications for sponsor-investigators. J. Investig. Med. 2009, 57, 688–694. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioeng. Transl. Med. 2021, 6, e10246. [Google Scholar] [CrossRef]
- Rasal, A.; Reddy, N.D. Chapter 14—Nano-pharmacokinetics preclinical to clinical translation. In Nano-Pharmacokinetics and Theranostics; Thorat, N.D., Kumar, N., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 273–288. [Google Scholar]
- Rasmussen, K.; Rauscher, H.; Kearns, P.; González, M.; Riego Sintes, J. Developing OECD test guidelines for regulatory testing of nanomaterials to ensure mutual acceptance of test data. Regul. Toxicol. Pharmacol. 2019, 104, 74–83. [Google Scholar] [CrossRef]
- Durán, C.E.; Cañás, M.; Urtasun, M.A.; Elseviers, M.; Andia, T.; Stichele, R.V.; Christiaens, T. Regulatory reliance to approve new medicinal products in Latin American and Caribbean countries. Rev. Panam. Salud. Pub. 2021, 45, 1–10. [Google Scholar] [CrossRef]
- Tobler, J.P.; Rocha, H.V.A. Bases regulatórias para a avaliação da segurança de medicamentos à base de nanotecnologia. Vigilância Sanitária Em Debate Soc. Ciência Tecnol. 2020, 8, 64–74. [Google Scholar] [CrossRef]
- Lemmens, T.; Vacaflor, C.H.; Gallagher, N.A.; Eagle, M.; Sarkar, N.; Cassiani, S.; Lori, J. Transparência nos ensaios clínicos nas Américas: É preciso coordenar as esferas reguladoras. Américas 2019, 43, e30. [Google Scholar] [CrossRef]
- IPRP. International Pharmaceutical Regulators Programme. Available online: https://www.iprp.global/home (accessed on 29 November 2021).
- Saldivar-Tanaka, L. Regulando la nanotecnología. Mundo Nano Rev. Interdiscip. Nanociencias Nanotecnol. 2018, 12, 1e–21e. [Google Scholar] [CrossRef]
- Soto-Vazquez, R.; Lau, E.Z.; López, L.A.M. Gobernanza de la nanomedicina: Una revisión sistemática. Mundo Nano Rev. Interdiscip. Nanociencias Nanotecnol. 2021, 15, 1e–25e. [Google Scholar] [CrossRef]











| Aspect | Food and Drug Agency United States of America | European Medicines Agency European Union | Ministry of Health, Labour and Welfare, MHLW |
|---|---|---|---|
| Biopharmaceutical application with innovative technologies with NPs | The Investigational New Drug (IND) application and New Drug Application (NDA) Reformulated Drug Products: Abbreviated New Drug Application (ANDA) | Investigational Medicinal Product Dossier (IMPD) | Investigational New Drug (IND) application or New Drug Application (NDA) under the format: Common Technical Document (CTD) |
| Nanomedicines Characterization Laboratory | US-NCL | EU-NCL | Research Center for Functional Materials |
| Guidelines for nanomedicines | Guidance for Industry on Drug Products, Including Biological Products that Contain Nanomaterials–Guidance for Industry Liposome Drug Products Guidance for Industry CDER and CBER Guidance | Reflection Paper on: Nanotechnology-Based Medicinal Products for Human Use Liposomal products Surface coatings Iron-based nano-colloidal products | Guideline for the Development of Liposome Drug Products Reflection paper on the development of block copolymer micelle medicinal products (MHLW/EMA) Reflection paper on silencing ribonucleic acid (siRNA) |
| References | [47,75,76] | [65,66,67,68,69] | [70,71,72] |
| Attribute | Technic | Standard | References |
|---|---|---|---|
| Size/Size distribution | Dynamic Light Scattering (DLS) | ISO 22412: 2017 | [133] |
| ASTM WK54872 * | [134] | ||
| NCL Joint Assay Protocol, PCC-1 | [135] | ||
| Electron Microscopy (TEM, SEM) | ISO 21363: 2020 | [136] | |
| ISO 19749: 2021 | [137] | ||
| Atomic Force Microscopy | ASTM E2859-11 (2017) | [138] | |
| Photon Correlation Spectroscopy (PCS) | ASTM E2490-09 (2021) | [139] | |
| Electrospray Differential Mobility Analysis (ES-DMA) | NCL Joint Assay Protocol, PCC-10 | [140] | |
| Differential Centrifugal Sedimentation (DCS) | ISO 13318-2: 2007 *** | [141] | |
| ISO 13318-3: 2004 ** | [142] | ||
| Size/Size distribution Particle by particle | Nanoparticle tracking analysis (NTA) | ASTM E2834-12 | [143] |
| Aperture/orifice tube method | ISO 13319-1: 2021 | [144] | |
| Tunable resistive pulse sensing (TRPS) | ISO/CD 13319-2* | [145] | |
| Nanoparticle Composition | Reversed-Phase High Performance Liquid Chromatography (RP-HPLC) | NCL Joint Assay Protocol, PCC-14 | [146] |
| Mass spectrometry (LC-MS) | ISO/TS 11251: 2019 | [147] | |
| Purity | Plasma mass spectrometry | ISO/TS 13278: 2017 | [148] |
| Chromatography | Scientific publications **** | - | |
| Nanoparticle concentration | Spectradyne nCS1TM | NCL Method PCC-20 | [149] |
| Surface Chemistry | Zeta Potential | NCL Method PCC-2 | [150] |
| ISO/TR 19997: 2018 | [151] | ||
| Asymmetric-Flow Field-Flow Fractionation (AF4) | ISO/TS 21362: 2018 | [152] | |
| NCL Method PCC-19 | [153] | ||
| Brunauer Emmet Teller | ISO/DIS 9277 * | [154] | |
| Aggregation/agglomeration status | DLS | ISO 22412: 2017 | [133] |
| Ultraviolet (UV)-visible (vis) | ISO/TS 17466: 2015 ** | [155] | |
| Particle Tracking Analysis | ISO/WD 19430 * | [156] | |
| Free and encapsulated drug | HPLC with UV-vis | ISO/TR 18196: 2016 | [157] |
| Stable Isotope Tracer Assay | |||
| Shape | Electron Microscopy | ISO 21363: 2020 | [136] |
| ISO 19749: 2021 | [137] | ||
| Solubility | pH, Electric conductivity | ISO/TR 13014: 2012/COR 1: 2012 ** | [158] |
| Approaches | Technique | Uses | Ref. |
|---|---|---|---|
| Bottom-up | Aerosol based processes | Useful for large-scale and multiscale nanoparticle design Aggregation control Size distribution control | [336] |
| Atomic or molecular condensation (gas condensation) | Used for metallic NPs It can be combined with chemical vapor deposition (CVD) Allows adjustment of physicochemical properties NPs formation is random | [337] | |
| Plasma processes | Reduces NPs agglomeration Produces NPs on a large scale Low cost and large volumes Used for NPs in cancer | [338] | |
| Chemical vapor deposition (CVD) | For inorganic NPs Reactor scale-up is easy Low production cost against large product volumes | [339] | |
| Liquid phase technique: Sol-gel | Generates solid colloidal particles in a range of less than 100 nm. They are formed from a liquid phase to gel Hydrolysis, condensation and polymerization phases Low-temperature synthesis and flexible design | [340] | |
| Top Down | Grinding and mechanical grinding | Used to produce a nanopowder It is difficult to control contamination and shape NP size can be controlled Easy to scale up | [341] |
| Microfluidization | Used for industrial production of liposomes High volume production Uses high pressure and size control Can damage product integrity | [342] | |
| Electrospray | Produces smaller and more uniform particle size Easy to control parameters Used for bulk production Most used for polymeric nanoparticles | [343] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, T.I.; Villacis-Aguirre, C.A.; López-Aguilar, K.V.; Santiago Padilla, L.; Altamirano, C.; Toledo, J.R.; Santiago Vispo, N. The Hitchhiker’s Guide to Human Therapeutic Nanoparticle Development. Pharmaceutics 2022, 14, 247. https://doi.org/10.3390/pharmaceutics14020247
Ramos TI, Villacis-Aguirre CA, López-Aguilar KV, Santiago Padilla L, Altamirano C, Toledo JR, Santiago Vispo N. The Hitchhiker’s Guide to Human Therapeutic Nanoparticle Development. Pharmaceutics. 2022; 14(2):247. https://doi.org/10.3390/pharmaceutics14020247
Chicago/Turabian StyleRamos, Thelvia I., Carlos A. Villacis-Aguirre, Katherine V. López-Aguilar, Leandro Santiago Padilla, Claudia Altamirano, Jorge R. Toledo, and Nelson Santiago Vispo. 2022. "The Hitchhiker’s Guide to Human Therapeutic Nanoparticle Development" Pharmaceutics 14, no. 2: 247. https://doi.org/10.3390/pharmaceutics14020247