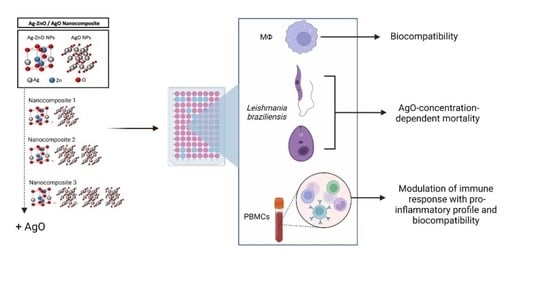
Development of Ag-ZnO/AgO Nanocomposites Effectives for Leishmania braziliensis Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of the Ag-ZnO/AgO Nanocomposites
2.2. RAW 264.7 and Leishmania braziliensis Cultivation
2.3. Anti-L. braziliensis Activity against Axenic Promastigotes, RAW 264.7 and Intracellular Amastigote
2.4. L. braziliensis Promastigote Growth Curve
2.5. Isolation and Treatment of Human Peripheral Blood Mononuclear Cells (PBMCs)
2.6. Immunophenotyping
2.7. Cytokines and Nitric Oxide (NO) Production
2.8. Statistical Analysis
3. Results
3.1. Structural and Morphological Properties of Ag-ZnO/AgO Nanocomposites
3.2. The NCPs Showed High Selectivity Index for L. braziliensis
3.3. ZnO nanocrystals, ZnO:9Ag and ZnO:11Ag NCPs Reduce the Proliferation of L. braziliensis Promastigotes
3.4. Ag-ZnO/AgO NCPs Reduce L. braziliensis Intracellular Amastigotes
3.5. Ag-ZnO/AgO Activate LTCD4+ and LTCD8+ and Induce Low Cytotoxic Levels at the Lowest Concentrations
3.6. Expression of TNFR1 and TNFR2
3.7. NPCs Differentially Regulate Immunoregulatory Molecules in CD4+ and CD8+ T Cells
3.8. NCPs Induce a Proinflammatory Cytokine Secretion Profile and Increase Nitric Oxide (NO)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobets, T.; Grekov, I.; Lipoldova, M. Leishmaniasis: Prevention, parasite detection and treatment. Curr. Med. Chem. 2012, 19, 1443–1474. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global leishmaniasis surveillance: 2019–2020, a baseline for the 2030 roadmap—Surveillance mondiale de la leishmaniose: 2019–2020, une période de référence pour la feuille de route à l’horizon 2030. Wkly. Epidemiol. Rec. 2021, 96, 401–419. [Google Scholar]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Narváez, F.J.; Vargas-González, A.; Canto-Lara, S.B.; Damián-Centeno, A.G. Clinical picture of cutaneous leishmaniases due to Leishmania (Leishmania) mexicana in the Yucatan peninsula, Mexico. Mem. Inst. Oswaldo Cruz. 2001, 96, 163–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reithinger, R.; Dujardin, J.C.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatami, A.; Firooz, A.; Gorouhi, F.; Khamesiour, A.; Dowlati, Y. Cutaneous leishmaniasis. Lancet Infect. Dis. 2008, 8, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Leishmaniasis 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 22 November 2022).
- Scott, P.; Novais, F.O. Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.I.; Brodskyn, C.I. The immunobiology of Leishmania braziliensis infection. Front Immunol. 2012, 3, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDC. Epidemiology & Risk Factors. 2020. Available online: https://www.cdc.gov/parasites/leishmaniasis/epi.html#print (accessed on 22 November 2022).
- Anversa, L.; Tiburcio, M.G.S.; Richini-Pereira, V.B.; Ramirez, L.E. Human leishmaniasis in Brazil: A general review. Rev. Assoc. Med. Bras. 2018, 64, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDC. Division of Parasitic Diseases and Malaria. 2022. Available online: https://www.cdc.gov/parasites/about/index.html (accessed on 22 November 2022).
- Aronson, N.; Herwaldt, B.L.; Libman, M.; Pearson, R.; Lopez-Velez, R.; Weina, P.; Carvalho, E.M.; Ephros, M.; Jeronimo, S.; Magill, A. Diagnosis and Treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin. Infect. Dis. 2016, 63, e202–e264. [Google Scholar] [CrossRef] [Green Version]
- Mitropoulos, P.; Konidas, P.; Durkin-Konidas, M. New World cutaneous leishmaniasis: Updated review of current and future diagnosis and treatment. J. Am. Acad. Dermatol. 2010, 63, 309–322. [Google Scholar] [CrossRef]
- Wijnant, G.-J.; Dumetz, F.; Dirkx, L.; Bulté, D.; Cuypers, B.; Van Bocxlaer, K.; Hendrickx, S. Tackling Drug Resistance and Other Causes of Treatment Failure in Leishmaniasis. Front. Trop. Dis. 2022, 3, 837460. [Google Scholar] [CrossRef]
- Sereno, D.; Maia, C.; Aït-Oudhia, K. Antimony resistance and environment: Elusive links to explore during Leishmania life cycle. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 200–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.T.; Musarrat, J.; Al-Khedhairy, A.A. Countering drug resistance, infectious diseases, and sepsis using metal and metal oxides nanoparticles: Current status. Colloids Surf. B Biointerfaces 2016, 146, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Khan, M.M.; Ahmad, J.; Alrokayan, S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomed. 2012, 7, 845–857. [Google Scholar]
- Gupta, J.; Safdari, H.A.; Hoque, M. Nanoparticle mediated cancer immunotherapy. Semin. Cancer Biol. 2021, 69, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Du, W. Zinc oxide nanoparticles (ZnO NPs) combined with cisplatin and gemcitabine inhibits tumor activity of NSCLC cells. Aging 2020, 12, 25767–25777. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, R.U.K.; Rama, A.; Govindan, I.; Naha, A. The purview of doped nanoparticles: Insights into their biomedical applications. OpenNano 2022, 8, 100070. [Google Scholar] [CrossRef]
- De Freitas Oliveira, J.W.; da Silva, M.F.A.; Damasceno, I.Z.; Rocha, H.A.O.; da Silva Júnior, A.A.; Silva, M.S. In Vitro Validation of Antiparasitic Activity of PLA-Nanoparticles of Sodium Diethyldithiocarbamate against Trypanosoma cruzi. Pharmaceutics 2022, 14, 497. [Google Scholar] [CrossRef]
- Souza, A.O.; Oliveira, J.W.d.F.; Moreno, C.J.G.; de Medeiros, M.J.C.; Fernandes-Negreiros, M.M.; Souza, F.R.M.; Pontes, D.L.; Silva, M.S.; Rocha, H.A.O. Silver Nanoparticles Containing Fucoidan Synthesized by Green Method Have Anti-Trypanosoma cruzi Activity. Nanomaterials 2022, 12, 2059. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Molefe, N.I.; Awakan, O.J.; Nwonuma, C.O.; Alejolowo, O.O.; Olaolu, T.; Maimako, R.F.; Suganuma, K.; Han, Y.; Kato, K. Metal nanoparticles restrict the growth of protozoan parasites. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S86–S94. [Google Scholar] [CrossRef] [Green Version]
- Alajmi, R.A.; Al-Megrin, W.A.; Metwally, D.; Al-Subaie, H.; Altamrah, N.; Barakat, A.M.; Moneim, A.E.A.; Al-Otaibi, T.T.; El-Khadragy, M. Anti-Toxoplasma activity of silver nanoparticles green synthesized with Phoenix dactylifera and Ziziphus spina-christi extracts which inhibits inflammation through liver regulation of cytokines in Balb/c mice. Biosci. Rep. 2019, 39, BSR20190379. [Google Scholar] [CrossRef] [Green Version]
- Do Carmo Neto, J.R.; Guerra, R.O.; Machado, J.R.; Silva, A.C.A.; da Silva, M.V. Antiprotozoal and Anthelmintic Activity of Zinc Oxide Nanoparticles. Curr. Med. Chem. 2022, 29, 2127–2141. [Google Scholar] [CrossRef]
- Saleem, K.; Khursheed, Z.; Hano, C.; Anjum, I.; Anjum, S. Applications of Nanomaterials in Leishmaniasis: A Focus on Recent Advances and Challenges. Nanomaterials 2019, 9, 1749. [Google Scholar] [CrossRef] [Green Version]
- De Souza, A.; Marins, D.S.S.; Mathias, S.L.; Monteiro, L.M.; Yukuyama, M.N.; Scarim, C.B.; Löbenberg, R.; Bou-Chacra, N.A. Promising nanotherapy in treating leishmaniasis. Int. J. Pharm. 2018, 547, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Gupta, S.S. Anti-leishmanial Nanotherapeutics: A Current Perspective. Curr. Drug Metab. 2019, 20, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.M.; Abo-Taleb, H.A.; Abdelaziz, A.M.; El-Tabakh, M.A.M.; El-Feky, M.M.M.; Abu-Elghait, M. Daphnia magna and Gammarus pulex, novel promising agents for biomedical and agricultural applications. Sci. Rep. 2022, 12, 13690. [Google Scholar] [CrossRef] [PubMed]
- Baranov, M.V.; Kumar, M.; Sacanna, S.; Thutupalli, S.; van den Bogaart, G. Modulation of Immune Responses by Particle Size and Shape. Front. Immunol. 2020, 11, 607945. [Google Scholar] [CrossRef] [PubMed]
- Pachaiappan, R.; Rajendran, S.; Show, P.L.; Manavalan, K.; Naushad, M. Metal/metal oxide nanocomposites for bactericidal effect: A review. Chemosphere 2021, 272, 128607. [Google Scholar] [CrossRef]
- Singh, S. Zinc oxide nanoparticles impacts: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef]
- Król-Górniak, A.; Rafińska, K.; Monedeiro, F.; Pomastowski, P.; Buszewski, B. Comparison Study of Cytotoxicity of Bare and Functionalized Zinc Oxide Nanoparticles. Int. J. Mol. Sci. 2021, 22, 9529. [Google Scholar] [CrossRef]
- Peña-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, Post-Treatment Recovery, and Selectivity Analysis of Naturally Occurring Podophyllotoxins from Bursera fagaroides var. fagaroides on Breast Cancer Cell Lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef] [Green Version]
- Yen, H.J.; Hsu, S.H.; Tsai, C.L. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small 2009, 5, 1553–1561. [Google Scholar] [CrossRef]
- Lappas, C.M. The immunomodulatory effects of titanium dioxide and silver nanoparticles. Food Chem. Toxicol. 2015, 85, 78–83. [Google Scholar] [CrossRef]
- Simón-Vázquez, R.; Lozano-Fernández, T.; Dávila-Grana, A.; González-Fernández, A. Metal oxide nanoparticles interact with immune cells and activate different cellular responses. Int. J. Nanomed. 2016, 11, 4657–4668. [Google Scholar] [CrossRef] [Green Version]
- Dimitriou, N.M.; Tsekenis, G.; Balanikas, E.C.; Pavlopoulou, A.; Mitsiogianni, M.; Mantso, T.; Pashos, G.; Boudouvis, A.G.; Lykakis, I.N.; Tsigaridas, G.; et al. Gold nanoparticles, radiations and the immune system: Current insights into the physical mechanisms and the biological interactions of this new alliance towards cancer therapy. Pharmacol. Ther. 2017, 178, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Bharat, T.C.; Shubham; Mondal, S.; Gupta, H.S.; Singh, P.K.; Das, A.K. Synthesis of Doped Zinc Oxide Nanoparticles: A Review. Mater. Today Proc. 2019, 11, 767–775. [Google Scholar] [CrossRef]
- Khan, Y.A.; Singh, B.R.; Ullah, R.; Shoeb, M.; Naqvi, A.H.; Abidi, S.M.A. Anthelmintic Effect of Biocompatible Zinc Oxide Nanoparticles (ZnO NPs) on Gigantocotyle explanatum, a Neglected Parasite of Indian Water Buffalo. PLoS ONE 2015, 10, e0133086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayyif, S.M.I.; Mohammed, H.B.; Curuțiu, C.; Bîrcă, A.C.; Grumezescu, A.M.; Vasile, B.; Dițu, L.M.; Lazăr, V.; Chifiriuc, M.C.; Mihăescu, G.; et al. ZnO Nanoparticles-Modified Dressings to Inhibit Wound Pathogens. Materials 2021, 14, 3084. [Google Scholar] [CrossRef]
- Majhi, R.K.; Mohanty, S.; Khan, M.I.; Mishra, A.; Brauner, A. Ag@ZnO Nanoparticles Induce Antimicrobial Peptides and Promote Migration and Antibacterial Activity of Keratinocytes. ACS Infect. Dis. 2021, 7, 2068–2072. [Google Scholar] [CrossRef]
- Reis, É.d.M.; de Rezende, A.A.A.; Santos, D.V.; de Oliveria, P.F.; Nicolella, H.D.; Tavares, D.C.; Silva, A.C.A.; Dantas, N.O.; Spanóa, M.A. Assessment of the genotoxic potential of two zinc oxide sources (amorphous and nanoparticles) using the in vitro micronucleus test and the in vivo wing somatic mutation and recombination test. Food Chem. Toxicol. 2015, 84, 55–63. [Google Scholar] [CrossRef]
- Murray, H. Susceptibility of leishmania to oxygen intermediates and killing by normal macrophages. J. Exp. Med. 1981, 153, 1302–1315. [Google Scholar] [CrossRef] [Green Version]
- Resta, R.; Thompson, L.F. T cell signalling through CD73. Cell. Signal. 1997, 9, 131–139. [Google Scholar] [CrossRef]
- Rodriguez-Pinto, D.; Saravia, N.G.; McMahon-Pratt, D. CD4 T cell activation by B cells in human Leishmania (Viannia) infection. BMC Infect. Dis. 2014, 14, 108. [Google Scholar] [CrossRef] [Green Version]
- Maspi, N.; Abdoli, A.; Ghaffarifar, F. Pro- and anti-inflammatory cytokines in cutaneous leishmaniasis: A review. Pathog. Glob. Health 2016, 110, 247–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amorim, C.F.; Novais, F.O.; Nguyen, B.T.; Misic, A.M.; Carvalho, L.P.; Carvalho, E.M.; Beiting, D.P.; Scott, P. Variable gene expression and parasite load predict treatment outcome in cutaneous leishmaniasis. Sci. Transl. Med. 2019, 11, eaax4204. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothi, P.C.; Cha, S.J.; Yang, I.J.; Sreekanth, T.V.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B 2015, 146, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Egui, A.; Ledesma, D.; Pérez-Antón, E.; Montoya, A.; Gómez, I.; Robledo, S.M.; Infante, J.J.; Vélez, I.D.; López, M.C.; Thomas, M.C. Phenotypic and Functional Profiles of Antigen-Specific CD4+ and CD8+ T Cells Associated with Infection Control in Patients with Cutaneous Leishmaniasis. Front. Cell. Infect. Microbiol. 2018, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Garcia de Moura, R.; Covre, L.P.; Fantecelle, C.H.; Gajardo, V.A.T.; Cunha, C.B.; Stringari, L.L.; Belew, A.T.; Daniel, C.B.; von Zeidler, S.V.; Tadokoro, C.E. PD-1 Blockade Modulates Functional Activities of Exhausted-Like T Cell in Patients with Cutaneous Leishmaniasis. Front. Immunol. 2021, 12, 632667. [Google Scholar] [CrossRef] [PubMed]
- Da-Cruz, A.M.; de Oliveira, M.P.; de Luca, P.M.; Mendonça, S.C.F.; Coutinho, S.G. Tumor necrosis factor-α in human American tegumentary leishmaniasis. Memórias Do Inst. Oswaldo Cruz 1996, 91, 225–229. [Google Scholar] [CrossRef]
- Saldanha, M.G.; Pagliari, C.; Queiroz, A.; Machado, P.R.L.; Carvalho, L.; Scott, P.; Carvalho, E.M.; Arruda, S. Tissue Damage in Human Cutaneous Leishmaniasis: Correlations Between Inflammatory Cells and Molecule Expression. Front. Cell. Infect. Microbiol. 2020, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.N.; Ribeiro, L.E.; Schrieffer, A.; Machado, P.; Carvalho, E.M.; Bacellar, O. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of human tegumentary leishmaniasis. Cytokine 2014, 66, 127–132. [Google Scholar] [CrossRef]








| Nanomaterial | Target Cell | CC50/IC50 (μg/mL) | Selectivity Index |
|---|---|---|---|
| ZnO nanocrystal | RAW 264.7 | 1232 | 1.33 |
| L.b | 927.9 | ||
| ZnO:5Ag NCPs | RAW 264.7 | 1330 | 3.35 |
| L.b | 397 | ||
| ZnO:9Ag NCPs | RAW 264.7 | 412.6 | 52.03 * |
| L.b | 7.93 | ||
| ZnO:11Ag NCPs | RAW 264.7 | 312.4 | 20.38 * |
| L.b | 15.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, R.M.; Obata, M.M.S.; Neto, J.R.d.C.; Guerra, R.O.; Borges, A.V.B.e.; Trevisan, R.O.; Ruiz, L.C.; Bernardi, J.d.M.; Oliveira-Scussel, A.C.d.M.; Vaz Tanaka, S.C.S.; et al. Development of Ag-ZnO/AgO Nanocomposites Effectives for Leishmania braziliensis Treatment. Pharmaceutics 2022, 14, 2642. https://doi.org/10.3390/pharmaceutics14122642
Barbosa RM, Obata MMS, Neto JRdC, Guerra RO, Borges AVBe, Trevisan RO, Ruiz LC, Bernardi JdM, Oliveira-Scussel ACdM, Vaz Tanaka SCS, et al. Development of Ag-ZnO/AgO Nanocomposites Effectives for Leishmania braziliensis Treatment. Pharmaceutics. 2022; 14(12):2642. https://doi.org/10.3390/pharmaceutics14122642
Chicago/Turabian StyleBarbosa, Rafaela Miranda, Malu Mateus Santos Obata, José Rodrigues do Carmo Neto, Rhanoica Oliveira Guerra, Anna Victória Bernardes e Borges, Rafael Obata Trevisan, Letícia Cirelli Ruiz, Júlia de Moura Bernardi, Ana Carolina de Morais Oliveira-Scussel, Sarah Cristina Sato Vaz Tanaka, and et al. 2022. "Development of Ag-ZnO/AgO Nanocomposites Effectives for Leishmania braziliensis Treatment" Pharmaceutics 14, no. 12: 2642. https://doi.org/10.3390/pharmaceutics14122642