Cytokine Therapy Combined with Nanomaterials Participates in Cancer Immunotherapy
Abstract
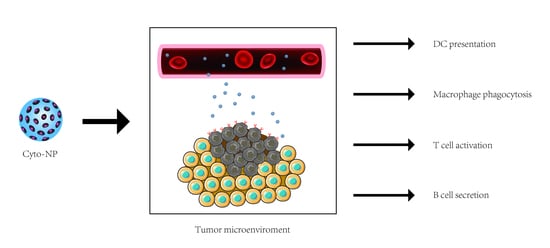
:1. Introduction
2. Organic Nanomaterials
2.1. Poly (Lactic-Co-Glycolic Acid)-Based Nanomaterials
2.2. Poly-γ-Glutamic Acid-Based Nanomaterials
2.3. β-Cyclodextrin-Based Nanomaterials
2.4. Chitosan-Based Nanomaterials
2.5. Polyethyleneimine-Based Nanomaterials
2.6. Liposomes-Based Nanomaterials
3. Inorganic Nanomaterials
3.1. Silica Nanoparticles
3.2. Magnetic Nanoparticles
3.3. Gold Nanoparticles
3.4. Calcium Carbonate/Calcium Phosphate Nanoparticles
4. Novel Nano Delivery Systems
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Wang, C.; Chen, G.; Hu, Q.; Gu, Z. Delivery strategies for immune checkpoint blockade. Adv. Healthc. Mater. 2018, 7, 1800424. [Google Scholar] [CrossRef] [PubMed]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oiseth, S.J.; Aziz, M.S. Cancer immunotherapy: A brief review of the history, possibilities, and challenges ahead. J. Cancer Metastasis Treat. 2017, 3, 250–261. [Google Scholar] [CrossRef]
- Fehleisen, F. Ueber die Züchtung der Erysipelkokken auf künstlichem Nährboden und ihre Übertragbarkeit auf den Menschen. Dtsch. Med. Wochenschr. 1882, 8, 553–554. [Google Scholar]
- Busch, W. Aus der Sitzung der medicinischen Section vom 13 November 1867. Berl. Klin. Wochenschr. 1868, 5, 137. [Google Scholar]
- Coley, W.B. The treatment of malignant tumors by repeated inoculations of erysipelas: With a report of ten original cases. 1. Am. J. Med. Sci. (1827–1924) 1893, 105, 487. [Google Scholar] [CrossRef]
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154. [Google Scholar]
- Decker, W.K.; da Silva, R.F.; Sanabria, M.H.; Angelo, L.S.; Guimarães, F.; Burt, B.M.; Kheradmand, F.; Paust, S. Cancer immunotherapy: Historical perspective of a clinical revolution and emerging preclinical animal models. Front. Immunol. 2017, 8, 829. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.; Mitchell, G.; Weiss, N. Cellular basis of the immunological defects in thymectomized mice. Nature 1967, 214, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Aulitzky, W.; Gastl, G.; Tilg, H.; Troppmair, J.; Leiter, E.; Geissler, D.; Flener, R.; Huber, C. Interferon-alpha in the treatment of hematologic neoplasms. Wien. Med. Wochenschr. (1946) 1986, 136, 172–181. [Google Scholar]
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.F.; Regan, M.M.; Clark, J.I.; Flaherty, L.E.; Weiss, G.R.; Logan, T.F.; Kirkwood, J.M.; Gordon, M.S.; Sosman, J.A.; Ernstoff, M.S.; et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2005, 23, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Dutcher, J.P.; Schwartzentruber, D.J.; Kaufman, H.L.; Agarwala, S.S.; Tarhini, A.A.; Lowder, J.N.; Atkins, M.B. High dose interleukin-2 (Aldesleukin)-expert consensus on best management practices-2014. J. Immunother. Cancer 2014, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Alspach, E.; Lussier, D.M.; Schreiber, R.D. Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb. Perspect. Biol. 2019, 11, a028480. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Su, M.; Liu, L.; Tang, Y.; Pan, Y.; Sun, J. Clinical Application of Cytokines in Cancer Immunotherapy. Drug Des Dev. Ther. 2021, 15, 2269–2287. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Suh, H.; Irvine, D.J. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.T.; Keller, S.; Manganiello, M.J.; Cheng, C.; Lee, C.-C.; Opara, C.; Convertine, A.; Stayton, P.S. pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano 2013, 7, 3912–3925. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; He, S.; Wang, Y. Toll-like receptor 7/8 agonist, R848, exhibits antitumoral effects in a breast cancer model. Mol. Med. Rep. 2015, 12, 3515–3520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, C.; Camps, M.; Li, T.; Chan, A.; Ossendorp, F.; Cruz, L. Co-delivery of immunomodulators in biodegradable nanoparticles improves therapeutic efficacy of cancer vaccines. Biomaterials 2019, 220, 119417. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, N.E.; Wen, S.; Driesschaert, B.; Eubank, T.D. Formulation and In Vitro Characterization of PLGA/PLGA-PEG Nanoparticles Loaded with Murine Granulocyte-Macrophage Colony-Stimulating Factor. AAPS PharmSciTech 2021, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Pinto, M.L.; Almeida, R.; Pereira, F.; Silva, A.M.; Pereira, C.L.; Santos, S.G.; Barbosa, M.A.; Gonçalves, R.M.; Oliveira, M.J. Chitosan/poly(γ-glutamic acid) nanoparticles incorporating IFN-γ for immune response modulation in the context of colorectal cancer. Biomater. Sci. 2019, 7, 3386–3403. [Google Scholar] [CrossRef]
- Kim, S.Y.; Noh, Y.W.; Kang, T.H.; Kim, J.E.; Kim, S.; Um, S.H.; Oh, D.B.; Park, Y.M.; Lim, Y.T. Synthetic vaccine nanoparticles target to lymph node triggering enhanced innate and adaptive antitumor immunity. Biomaterials 2017, 130, 56–66. [Google Scholar] [CrossRef]
- Li, J.M.; Wang, Y.Y.; Zhang, W.; Su, H.; Ji, L.N.; Mao, Z.W. Low-weight polyethylenimine cross-linked 2-hydroxypopyl-β-cyclodextrin and folic acid as an efficient and nontoxic siRNA carrier for gene silencing and tumor inhibition by VEGF siRNA. Int. J. Nanomed. 2013, 8, 2101–2117. [Google Scholar] [CrossRef] [Green Version]
- Nahaei, M.; Valizadeh, H.; Baradaran, B.; Nahaei, M.R.; Asgari, D.; Hallaj-Nezhadi, S.; Dastmalchi, S.; Lotfipour, F. Preparation and characterization of chitosan/β-cyclodextrin nanoparticles containing plasmid DNA encoding interleukin-12. Drug Res. 2013, 63, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Clemente, N.; Boggio, E.; Gigliotti, L.C.; Raineri, D.; Ferrara, B.; Miglio, G.; Argenziano, M.; Chiocchetti, A.; Cappellano, G.; Trotta, F.; et al. Immunotherapy of experimental melanoma with ICOS-Fc loaded in biocompatible and biodegradable nanoparticles. J. Control. Release 2020, 320, 112–124. [Google Scholar] [CrossRef]
- Rodell, C.B.; Arlauckas, S.P.; Cuccarese, M.F.; Garris, C.S.; Li, R.; Ahmed, M.S.; Kohler, R.H.; Pittet, M.J.; Weissleder, R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588. [Google Scholar] [CrossRef]
- Seo, S.H.; Han, H.D.; Noh, K.H.; Kim, T.W.; Son, S.W. Chitosan hydrogel containing GMCSF and a cancer drug exerts synergistic anti-tumor effects via the induction of CD8+ T cell-mediated anti-tumor immunity. Clin. Exp. Metastasis 2009, 26, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Guo, L.; Gu, X.; Zhang, B.; Hu, X.; Zhang, J.; Chen, J.; Wang, Y.; Chen, C.; Gao, B.; et al. Prevention of colorectal cancer liver metastasis by exploiting liver immunity via chitosan-TPP/nanoparticles formulated with IL-12. Biomaterials 2012, 33, 3909–3918. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.; Park, W.; Kim, D.; Fahmy, T.M.; Na, K. A self-assembled polymeric micellar immunomodulator for cancer treatment based on cationic amphiphilic polymers. Biomaterials 2014, 35, 9912–9919. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, Y.; Peng, K.; Wang, Q.; Hong, X.; Li, H.; Fan, G.; Zhang, Z.; Gong, T.; Sun, X. Combined delivery of a TGF-beta inhibitor and an adenoviral vector expressing interleukin-12 potentiates cancer immunotherapy. Acta Biomater. 2017, 61, 114–123. [Google Scholar] [CrossRef]
- Kwong, B.; Gai, S.A.; Elkhader, J.; Wittrup, K.D.; Irvine, D.J. Localized Immunotherapy via Liposome-Anchored Anti-CD137 + IL-2 Prevents Lethal Toxicity and Elicits Local and Systemic Antitumor Immunity. Cancer Res. 2013, 73, 1547–1558. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Tang, L.; Mabardi, L.; Kumari, S.; Irvine, D.J. Enhancing Adoptive Cell Therapy of Cancer through Targeted Delivery of Small-Molecule Immunomodulators to Internalizing or Noninternalizing Receptors. ACS Nano 2017, 11, 3089–3100. [Google Scholar] [CrossRef] [Green Version]
- Kong, M.; Tang, J.; Qiao, Q.; Wu, T.; Qi, Y.; Tan, S.; Gao, X.; Zhang, Z. Biodegradable Hollow Mesoporous Silica Nanoparticles for Regulating Tumor Microenvironment and Enhancing Antitumor Efficiency. Theranostics 2017, 7, 3276–3292. [Google Scholar] [CrossRef]
- Wan, Y.; Yu, W.; Li, J.; Peng, N.; Ding, X.; Wang, Y.; Zou, T.; Cheng, Y.; Liu, Y. Multi-functional carboxymethyl chitin-based nanoparticles for modulation of tumor-associated macrophage polarity. Carbohydr. Polym. 2021, 267, 118245. [Google Scholar] [CrossRef]
- Mejias, R.; Perez-Yague, S.; Gutierrez, L.; Cabrera, L.I.; Spada, R.; Acedo, P.; Serna, C.J.; Lazaro, F.J.; Villanueva, A.; Morales Mdel, P.; et al. Dimercaptosuccinic acid-coated magnetite nanoparticles for magnetically guided in vivo delivery of interferon gamma for cancer immunotherapy. Biomaterials 2011, 32, 2938–2952. [Google Scholar] [CrossRef]
- Hu, B.; Du, H.J.; Yan, G.P.; Zhuo, R.X.; Wu, Y.; Fan, C.L. Magnetic polycarbonate microspheres for tumor-targeted delivery of tumor necrosis factor. Drug Deliv. 2014, 21, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Tong, J.; Wu, J.; Xu, X.; Wu, S.; Tan, B.; Shi, M.; Wang, J.; Zhao, W.; Jiang, H.; et al. Preclinical evaluation of recombinant human IFNalpha2b-containing magnetoliposomes for treating hepatocellular carcinoma. Int. J. Nanomed. 2014, 9, 4533–4550. [Google Scholar] [CrossRef] [Green Version]
- Curnis, F.; Fiocchi, M.; Sacchi, A.; Gori, A.; Gasparri, A.; Corti, A. NGR-tagged nano-gold: A new CD13-selective carrier for cytokine delivery to tumors. Nano Res. 2016, 9, 1393–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohseni, N.; Sarvestani, F.S.; Ardestani, M.S.; Kazemi-Lomedasht, F.; Ghorbani, M. Inhibitory effect of gold nanoparticles conjugated with interferon gamma and methionine on breast cancer cell line. Asian Pac. J. Trop. Biomed. 2016, 6, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Yu, L.; Gu, P.; Bo, R.; Wusiman, A.; Liu, J.; Hu, Y.; Wang, D. Preparation of lentinan-calcium carbonate microspheres and their application as vaccine adjuvants. Carbohydr. Polym. 2020, 245, 116520. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Cong, X.; Feng, L.; Chen, H.; Wang, J.; Wu, C.; Liu, K.; Xiao, C.; Yang, Y.G.; Sun, T. Intratumoral delivery of M-CSF by calcium crosslinked polymer micelles enhances cancer immunotherapy. Biomater. Sci. 2019, 7, 2769–2776. [Google Scholar] [CrossRef] [PubMed]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef]
- Lü, J.M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021, 6, 346–360. [Google Scholar] [CrossRef]
- Vasir, J.K.; Labhasetwar, V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv. Drug Deliv. Rev. 2007, 59, 718–728. [Google Scholar] [CrossRef] [Green Version]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Babu, V.R.; Rangaswamy, V.; Patel, P.; Aminabhavi, T.M. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J. Control. Release 2008, 125, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Qodratnama, R.; Serino, L.P.; Cox, H.C.; Qutachi, O.; White, L.J. Formulations for modulation of protein release from large-size PLGA microparticles for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 47, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Maravajjala, K.S.; Swetha, K.L.; Roy, A. pH-Responsive Nanoparticles for Multidimensional Combined Chemo-Immunotherapy of Cancer. J. Pharm. Sci. 2022, 111, 2353–2368. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, W.-L.; Kheirolomoom, A.; Fite, B.Z.; Wu, B.; Lau, K.; Baikoghli, M.; Raie, M.N.; Tumbale, S.K.; Foiret, J. Development of thermosensitive resiquimod-loaded liposomes for enhanced cancer immunotherapy. J. Control. Release 2021, 330, 1080–1094. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lepland, A.; Scodeller, P.; Fontana, F.; Torrieri, G.; Tiboni, M.; Shahbazi, M.A.; Casettari, L.; Kostiainen, M.A.; Hirvonen, J. Peptide-guided resiquimod-loaded lignin nanoparticles convert tumor-associated macrophages from M2 to M1 phenotype for enhanced chemotherapy. Acta Biomater. 2021, 133, 231–243. [Google Scholar] [CrossRef]
- Chen, P.-M.; Pan, W.-Y.; Wu, C.-Y.; Yeh, C.-Y.; Korupalli, C.; Luo, P.-K.; Chou, C.-J.; Chia, W.-T.; Sung, H.-W. Modulation of tumor microenvironment using a TLR-7/8 agonist-loaded nanoparticle system that exerts low-temperature hyperthermia and immunotherapy for in situ cancer vaccination. Biomaterials 2020, 230, 119629. [Google Scholar] [CrossRef]
- Tang, X.D.; Lu, K.L.; Yu, J.; Du, H.J.; Fan, C.Q.; Chen, L. In vitro and in vivo evaluation of DC-targeting PLGA nanoparticles encapsulating heparanase CD4(+) and CD8(+) T-cell epitopes for cancer immunotherapy. Cancer Immunol. Immunother. 2022, 71, 2969–2983. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chung, C.K.; Chen, C.T.; Liang, H.F.; Chen, S.C.; Sung, H.W. Preparation of nanoparticles composed of chitosan/poly-gamma-glutamic acid and evaluation of their permeability through Caco-2 cells. Biomacromolecules 2005, 6, 1104–1112. [Google Scholar] [CrossRef]
- Luo, Z.; Guo, Y.; Liu, J.; Qiu, H.; Zhao, M.; Zou, W.; Li, S. Microbial synthesis of poly-γ-glutamic acid: Current progress, challenges, and future perspectives. Biotechnol. Biofuels 2016, 9, 134. [Google Scholar] [CrossRef] [Green Version]
- Ogunleye, A.; Bhat, A.; Irorere, V.U.; Hill, D.; Williams, C.; Radecka, I. Poly-γ-glutamic acid: Production, properties and applications. Microbiology 2015, 161, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Camero, G.; Congregado, F.; Bou, J.J.; Muñoz-Guerra, S. Biosynthesis and ultrasonic degradation of bacterial poly(gamma-glutamic acid). Biotechnol. Bioeng 1999, 63, 110–115. [Google Scholar] [CrossRef]
- Shih, I.L.; Van, Y.T.; Yeh, L.C.; Lin, H.G.; Chang, Y.N. Production of a biopolymer flocculant from Bacillus licheniformis and its flocculation properties. Bioresour. Technol. 2001, 78, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Fan, L.; Zhao, M.; Qiu, Y.; Zhao, L. Enhanced Low Molecular Weight Poly-γ-Glutamic Acid Production in Recombinant Bacillus subtilis 1A751 with Zinc Ion. Appl. Biochem. Biotechnol. 2019, 189, 411–423. [Google Scholar] [CrossRef]
- Broos, S.; Sandin, L.C.; Apel, J.; Tötterman, T.H.; Akagi, T.; Akashi, M.; Borrebaeck, C.A.; Ellmark, P.; Lindstedt, M. Synergistic augmentation of CD40-mediated activation of antigen-presenting cells by amphiphilic poly(γ-glutamic acid) nanoparticles. Biomaterials 2012, 33, 6230–6239. [Google Scholar] [CrossRef]
- Li, K.; Li, D.; Zhao, L.; Chang, Y.; Zhang, Y.; Cui, Y.; Zhang, Z. Calcium-mineralized polypeptide nanoparticle for intracellular drug delivery in osteosarcoma chemotherapy. Bioact. Mater. 2020, 5, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Pinto, M.L.; Silva, A.M.; Pereira, C.L.; Teixeira, G.Q.; Gomez-Lazaro, M.; Santos, S.G.; Barbosa, M.A.; Gonçalves, R.M.; Oliveira, M.J. Pro-inflammatory chitosan/poly(γ-glutamic acid) nanoparticles modulate human antigen-presenting cells phenotype and revert their pro-invasive capacity. Acta Biomater. 2017, 63, 96–109. [Google Scholar] [CrossRef]
- Mealy, J.E.; Rodell, C.B.; Burdick, J.A. Sustained Small Molecule Delivery from Injectable Hyaluronic Acid Hydrogels through Host-Guest Mediated Retention. J. Mater. Chem. B 2015, 3, 8010–8019. [Google Scholar] [CrossRef] [Green Version]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Watanabe, A.; Nishida, S.; Burcu, T.; Shibahara, T.; Kusakabe, T.; Kuroda, E.; Ishii, K.J.; Kumanogoh, A. Safety and immunogenicity of a quadrivalent seasonal influenza vaccine adjuvanted with hydroxypropyl-β-cyclodextrin: A phase 1 clinical trial. Vaccine 2022, 40, 4150–4159. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Jiang, X.; Yang, T.; Xu, H.; Xie, Q.; Hu, M.; Yang, C.; Kong, L.; Zhang, Z. Enhancing cancer chemo-immunotherapy by biomimetic nanogel with tumor targeting capacity and rapid drug-releasing in tumor microenvironment. Acta Pharm. Sin. B 2022, 12, 2550–2567. [Google Scholar] [CrossRef] [PubMed]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Hassainia, A.; Satha, H.; Boufi, S. Chitin from Agaricus bisporus: Extraction and characterization. Int. J. Biol. Macromol. 2018, 117, 1334–1342. [Google Scholar] [CrossRef]
- Liu, X.; Hao, M.; Chen, Z.; Zhang, T.; Huang, J.; Dai, J.; Zhang, Z. 3D bioprinted neural tissue constructs for spinal cord injury repair. Biomaterials 2021, 272, 120771. [Google Scholar] [CrossRef]
- Lima, B.V.; Oliveira, M.J.; Barbosa, M.A.; Gonçalves, R.M.; Castro, F. Immunomodulatory potential of chitosan-based materials for cancer therapy: A systematic review of in vitro, in vivo and clinical studies. Biomater. Sci. 2021, 9, 3209–3227. [Google Scholar] [CrossRef]
- Moran, H.B.T.; Turley, J.L.; Andersson, M.; Lavelle, E.C. Immunomodulatory properties of chitosan polymers. Biomaterials 2018, 184, 1–9. [Google Scholar] [CrossRef]
- Gong, Y.; Tao, L.; Wang, F.; Liu, W.; Jing, L.; Liu, D.; Hu, S.; Xie, Y.; Zhou, N. Chitosan as an adjuvant for a Helicobacter pylori therapeutic vaccine. Mol. Med. Rep. 2015, 12, 4123–4132. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.; Gupta, M.; Gupta, V.; Gogoi, H.; Bhatnagar, R. Novel application of trimethyl chitosan as an adjuvant in vaccine delivery. Int. J. Nanomed. 2018, 13, 7959–7970. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.S.; Xu, Y.L.; Zou, X.T.; Xu, Z.R. Chitosan nanoparticles act as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Mar. Drugs 2011, 9, 1038–1055. [Google Scholar] [CrossRef]
- Wu, J.; Tang, C.; Yin, C. Co-delivery of doxorubicin and interleukin-2 via chitosan based nanoparticles for enhanced antitumor efficacy. Acta Biomater. 2017, 47, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Jie, L.; Yongqi, W.; Weiming, X.; Juqun, X.; Yanbing, D.; Li, Q.; Xingyuan, P.; Mingchun, J.; Weijuan, G. Delivery of human NKG2D-IL-15 fusion gene by chitosan nanoparticles to enhance antitumor immunity. Biochem. Biophys. Res. Commun. 2015, 463, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Han, S.; Ding, S.; Xiao, W.; Ding, Y.; Qian, L.; Wang, C.; Gong, W. Chitosan nanoparticle-based delivery of fused NKG2D-IL-21 gene suppresses colon cancer growth in mice. Int. J. Nanomed. 2017, 12, 3095–3107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, M.; Li, P. Amine-containing core-shell nanoparticles as potential drug carriers for intracellular delivery. J. Biomed. Mater. Res. A 2007, 80, 184–193. [Google Scholar] [CrossRef]
- Pimpha, N.; Sunintaboon, P.; Inphonlek, S.; Tabata, Y. Gene delivery efficacy of polyethyleneimine-introduced chitosan shell/poly(methyl methacrylate) core nanoparticles for rat mesenchymal stem cells. J. Biomater. Sci. Polym. Ed. 2010, 21, 205–223. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Li, J.; Zhang, Y.; Li, Y.; Shen, G.; Zhu, J.; Tao, J. Polyethylenimine-based micro/nanoparticles as vaccine adjuvants. Int. J. Nanomed. 2017, 12, 5443–5460. [Google Scholar] [CrossRef] [Green Version]
- Awad, N.S.; Paul, V.; Mahmoud, M.S.; Al Sawaftah, N.M.; Kawak, P.S.; Al Sayah, M.H.; Husseini, G.A. Effect of Pegylation and Targeting Moieties on the Ultrasound-Mediated Drug Release from Liposomes. ACS Biomater. Sci. Eng. 2020, 6, 48–57. [Google Scholar] [CrossRef]
- Fernandez, M.F.; Qiao, G.; Tulla, K.; Prabhakar, B.S.; Maker, A.V. Combination Immunotherapy With LIGHT and Interleukin-2 Increases CD8 Central Memory T-Cells In Vivo. J. Surg. Res. 2021, 263, 44–52. [Google Scholar] [CrossRef]
- Liu, J.Q.; Zhang, C.; Zhang, X.; Yan, J.; Zeng, C.; Talebian, F.; Lynch, K.; Zhao, W.; Hou, X.; Du, S.; et al. Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J. Control. Release 2022, 345, 306–313. [Google Scholar] [CrossRef]
- Dormer, K.; Seeney, C.; Lewelling, K.; Lian, G.; Gibson, D.; Johnson, M. Epithelial internalization of superparamagnetic nanoparticles and response to external magnetic field. Biomaterials 2005, 26, 2061–2072. [Google Scholar] [CrossRef]
- Allouche, J.; Boissière, M.; Hélary, C.; Livage, J.; Coradin, T. Biomimetic core–shell gelatine/silica nanoparticles: A new example of biopolymer-based nanocomposites. J. Mater. Chem. 2006, 16, 3120–3125. [Google Scholar] [CrossRef]
- Huo, Q.; Liu, J.; Wang, L.Q.; Jiang, Y.; Lambert, T.N.; Fang, E. A new class of silica cross-linked micellar core-shell nanoparticles. J. Am. Chem. Soc. 2006, 128, 6447–6453. [Google Scholar] [CrossRef] [PubMed]
- Ghaferi, M.; Koohi Moftakhari Esfahani, M.; Raza, A.; Al Harthi, S.; Ebrahimi Shahmabadi, H.; Alavi, S.E. Mesoporous silica nanoparticles: Synthesis methods and their therapeutic use-recent advances. J. Drug Target. 2021, 29, 131–154. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Liu, H.; Fu, C.; Li, L.; Chen, D.; Zhang, Y.; Tang, F. Silica nanorattle with enhanced protein loading: A potential vaccine adjuvant. J. Colloid Interface Sci. 2013, 400, 168–174. [Google Scholar] [CrossRef]
- Choi, E.W.; Shin, I.S.; Chae, Y.J.; Koo, H.C.; Lee, J.H.; Chung, T.H.; Park, Y.H.; Kim, D.Y.; Hwang, C.Y.; Lee, C.W.; et al. Effects of GM-CSF gene transfer using silica-nanoparticles as a vehicle on white blood cell production in dogs. Exp. Hematol. 2008, 36, 807–815. [Google Scholar] [CrossRef]
- Gavilán, H.; Avugadda, S.K.; Fernández-Cabada, T.; Soni, N.; Cassani, M.; Mai, B.T.; Chantrell, R.; Pellegrino, T. Magnetic nanoparticles and clusters for magnetic hyperthermia: Optimizing their heat performance and developing combinatorial therapies to tackle cancer. Chem. Soc. Rev. 2021, 50, 11614–11667. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Rescignano, N.; Kenny, J.M. 8-Stimuli-responsive core-shell nanoparticles. In Core-Shell Nanostructures for Drug Delivery and Theranostics; Focarete, M.L., Tampieri, A., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 245–258. [Google Scholar]
- Zhao, J.; Friedrich, B. Synthesis of Gold Nanoparticles via Chemical Reduction Method. In Proceedings of the Nanocon, Brno, Czech Republic, 6–14 October 2015. [Google Scholar]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Curnis, F.; Sacchi, A.; Longhi, R.; Colombo, B.; Gasparri, A.; Corti, A. IsoDGR-tagged albumin: A new alphavbeta3 selective carrier for nanodrug delivery to tumors. Small 2013, 9, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, A.M.; Sacchi, A.; Basso, V.; Cortesi, F.; Freschi, M.; Rrapaj, E.; Bellone, M.; Casorati, G.; Dellabona, P.; Mondino, A.; et al. Boosting Interleukin-12 Antitumor Activity and Synergism with Immunotherapy by Targeted Delivery with isoDGR-Tagged Nanogold. Small 2019, 15, e1903462. [Google Scholar] [CrossRef] [Green Version]
- Donatan, S.; Yashchenok, A.; Khan, N.; Parakhonskiy, B.; Cocquyt, M.; Pinchasik, B.E.; Khalenkow, D.; Möhwald, H.; Konrad, M.; Skirtach, A. Loading Capacity versus Enzyme Activity in Anisotropic and Spherical Calcium Carbonate Microparticles. ACS Appl. Mater. Interfaces 2016, 8, 14284–14292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Sun, H.Y.; Wang, Z.C.; Xu, X.D.; Song, J.C.; Gong, Z.J. Fabrication of Alginate/Calcium Carbonate Hybrid Microparticles for Synergistic Drug Delivery. Chemotherapy 2016, 61, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, S.; Qin, Y.; Fan, F.; Zhang, Z.; Huang, C.; Ji, W.; Lu, L.; Wang, C.; Sun, H.; et al. Targeted Codelivery of an Antigen and Dual Agonists by Hybrid Nanoparticles for Enhanced Cancer Immunotherapy. Nano Lett. 2019, 19, 4237–4249. [Google Scholar] [CrossRef]
- Gao, Y.; Ouyang, Z.; Yang, C.; Song, C.; Jiang, C.; Song, S.; Shen, M.; Shi, X. Overcoming T Cell Exhaustion via Immune Checkpoint Modulation with a Dendrimer-Based Hybrid Nanocomplex. Adv. Heal. Mater. 2021, 10, e2100833. [Google Scholar] [CrossRef]
- Hou, L.; Tian, C.; Yan, Y.; Zhang, L.; Zhang, H.; Zhang, Z. Manganese-Based Nanoactivator Optimizes Cancer Immunotherapy via Enhancing Innate Immunity. ACS Nano 2020, 14, 3927–3940. [Google Scholar] [CrossRef]
- Hu, C.M.; Fang, R.H.; Luk, B.T.; Chen, K.N.; Carpenter, C.; Gao, W.; Zhang, K.; Zhang, L. ‘Marker-of-self’ functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale 2013, 5, 2664–2668. [Google Scholar] [CrossRef] [Green Version]
- Fang, R.H.; Hu, C.M.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, Y.; Feng, N. Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110298. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, P.; Luo, Z.; Zheng, M.; Tian, H.; Gong, P.; Gao, G.; Pan, H.; Liu, L.; Ma, A.; et al. Cancer Cell Membrane-Biomimetic Nanoparticles for Homologous-Targeting Dual-Modal Imaging and Photothermal Therapy. ACS Nano 2016, 10, 10049–10057. [Google Scholar] [CrossRef] [PubMed]
- Kunde, S.S.; Wairkar, S. Platelet membrane camouflaged nanoparticles: Biomimetic architecture for targeted therapy. Int J Pharm 2021, 598, 120395. [Google Scholar] [CrossRef]
- Chu, D.; Dong, X.; Shi, X.; Zhang, C.; Wang, Z. Neutrophil-Based Drug Delivery Systems. Adv. Mater. 2018, 30, e1706245. [Google Scholar] [CrossRef] [PubMed]
- Luk, B.T.; Zhang, L. Cell membrane-camouflaged nanoparticles for drug delivery. J. Control. Release 2015, 220, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Wang, C.; Liu, Z. Red Blood Cells as Smart Delivery Systems. Bioconjug. Chem. 2018, 29, 852–860. [Google Scholar] [CrossRef]
- Ortiz-Otero, N.; Mohamed, Z.; King, M.R. Platelet-Based Drug Delivery for Cancer Applications. Adv. Exp. Med. Biol. 2018, 1092, 235–251. [Google Scholar] [CrossRef]
- Maeding, N.; Verwanger, T.; Krammer, B. Boosting tumor-specific immunity using PDT. Cancers 2016, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Kabingu, E.; Vaughan, L.; Owczarczak, B.; Ramsey, K.; Gollnick, S. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br. J. Cancer 2007, 96, 1839–1848. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Gao, D.; Gao, L.; Lai, J.; Zhang, C.; Zhao, Y.; Zhong, L.; Jia, B.; Wang, F.; Chen, X. Inhibiting metastasis and preventing tumor relapse by triggering host immunity with tumor-targeted photodynamic therapy using photosensitizer-loaded functional nanographenes. ACS Nano 2017, 11, 10147–10158. [Google Scholar] [CrossRef] [PubMed]
- Facciabene, A.; Peng, X.; Hagemann, I.S.; Balint, K.; Barchetti, A.; Wang, L.-P.; Gimotty, P.A.; Gilks, C.B.; Lal, P.; Zhang, L. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 2011, 475, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xu, L.; Chao, Y.; Xu, J.; Sun, X.; Wu, Y.; Peng, R.; Liu, Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorusso, D.; Di Stefano, A.; Carone, V.; Fagotti, A.; Pisconti, S.; Scambia, G. Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia (‘hand-foot’ syndrome). Ann. Oncol. 2007, 18, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Ragelle, H.; Danhier, F.; Préat, V.; Langer, R.; Anderson, D.G. Nanoparticle-based drug delivery systems: A commercial and regulatory outlook as the field matures. Expert Opin. Drug Deliv. 2017, 14, 851–864. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Zucker, D.; Marcus, D.; Barenholz, Y.; Goldblum, A. Liposome drugs’ loading efficiency: A working model based on loading conditions and drug’s physicochemical properties. J. Control. Release 2009, 139, 73–80. [Google Scholar] [CrossRef]
- Hu, Q.; Shang, L.; Wang, M.; Tu, K.; Hu, M.; Yu, Y.; Xu, M.; Kong, L.; Guo, Y.; Zhang, Z. Co-Delivery of Paclitaxel and Interleukin-12 Regulating Tumor Microenvironment for Cancer Immunochemotherapy. Adv. Heal. Mater. 2020, 9, e1901858. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Zhang, L.; Huang, L. Nanoparticle-delivered transforming growth factor-β siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano 2014, 8, 3636–3645. [Google Scholar] [CrossRef]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef]
- Yoshida, Y.; Naito, M.; Yamada, T.; Aisu, N.; Kojima, D.; Mera, T.; Tanaka, T.; Naito, K.; Yasumoto, K.; Kamigaki, T.; et al. Clinical Study on the Medical Value of Combination Therapy Involving Adoptive Immunotherapy and Chemotherapy for Stage IV Colorectal Cancer (COMVI Study). Anticancer Res. 2017, 37, 3941–3946. [Google Scholar] [CrossRef] [PubMed]
- Baldo, B.A. Side effects of cytokines approved for therapy. Drug Saf. 2014, 37, 921–943. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, X.; Liang, X.; Yang, J.; Zhang, C.; Kong, D.; Wang, W. Nano-, micro-, and macroscale drug delivery systems for cancer immunot herapy. Acta Biomater. 2019, 85, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Chu, Q.; Liu, Y.; Zhang, N. A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy. Nano Micro Lett. 2020, 12, 142. [Google Scholar] [CrossRef]



| Nanomaterials | Cytokines | References | |
|---|---|---|---|
| Organic | PLGA-based nanomaterials | TNF-α, IL-6, IFN-α,GM-CSF | [22,23,24] |
| Poly-γ-glutamic acid-based nanomaterials | IL-10, IL-12, IL-6,TNF-α, IFN-γ | [25,26] | |
| β-Cyclodextrin-based nanomaterials | VEGF, IL-10, IL-12 | [27,28,29,30] | |
| Chitosan-based nanomaterials | IL-2, IL-12, IL-15, IL-21 | [31,32] | |
| Polyethyleneimine-based nanomaterials | IL-6, TNF-α, IL-12, IFN-γ | [33,34] | |
| Liposomes-based nanomaterials | IL-2, TGF-β | [35,36] | |
| Inorganic | Silica nanoparticles | IL-2, IFN-γ, IL-12 | [37,38] |
| Magnetic nanoparticles | IFN-γ, TNF-α, IFN-α | [39,40,41] | |
| Gold nanoparticles | TNF-α, IFN-γ | [39,42,43] | |
| Calcium carbonate/Calcium phosphate nanoparticles | IL-2, IL-4, M-CSF | [42,44,45] |
| Type | Chemical Structure | Properties |
|---|---|---|
| Chitosan |  | Nonimmunogenic; Biocompatible; Biodegradable; Mucoadhesion; |
| Trimethyl chitosan (TMC) |  | Positively charged; Penetration-enhancement; Mucoadhesion; |
| Carboxymethyl chitosan (CMC) |  | pH-dependent water solubility; Adhesion and absorption enhancement; Antibacterial and antioxidant activities; |
| Thiolated chitosan (TC) |  | Easily endocytosed by cells; Hydrophilic; In situ relatability; Mechanical stability; |
| Glycated chitosan (GC) |  | Immune-enhancing; Hydrophilic; Noncytotoxic; |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, H.; Ma, S.; Zhao, D.; Zhao, W.; Cui, Y.; Hua, Y.; Zhang, Z. Cytokine Therapy Combined with Nanomaterials Participates in Cancer Immunotherapy. Pharmaceutics 2022, 14, 2606. https://doi.org/10.3390/pharmaceutics14122606
Lian H, Ma S, Zhao D, Zhao W, Cui Y, Hua Y, Zhang Z. Cytokine Therapy Combined with Nanomaterials Participates in Cancer Immunotherapy. Pharmaceutics. 2022; 14(12):2606. https://doi.org/10.3390/pharmaceutics14122606
Chicago/Turabian StyleLian, Heping, Shuang Ma, Duoyi Zhao, Wei Zhao, Yan Cui, Yingqi Hua, and Zhiyu Zhang. 2022. "Cytokine Therapy Combined with Nanomaterials Participates in Cancer Immunotherapy" Pharmaceutics 14, no. 12: 2606. https://doi.org/10.3390/pharmaceutics14122606