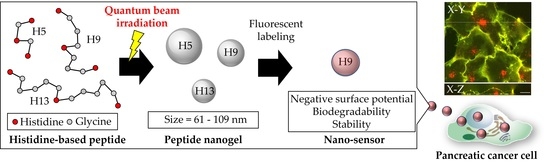
Synthesis of Small Peptide Nanogels Using Radiation Crosslinking as a Platform for Nano-Imaging Agents for Pancreatic Cancer Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation and Irradiation of the Aqueous Synthetic Peptide Solution
2.3. Pulse Radiolysis Study
2.4. Molecular Orbital Calculations and Kinetics Simulations
2.5. Dynamic Light Scattering (DLS) and Electrophoretic Light Scattering (ELS) Measurements
2.6. Biodegradability
2.7. Cellular Uptake Tests
2.8. Statistical Analysis
3. Results
3.1. Radiation Crosslinking of Peptides with Different Chain Lengths in Water
3.2. Aqueous Peptide Solution Kinetics after Ionizing Radiation
 OH + e−aq + H
OH + e−aq + H
3.3. Simulation of Radiation Crosslinking of Peptides in Water by Ionizing Radiation
| Peptide | Rate Constant with Hydroxyl Radicals (mol−1 L s−1) |
|---|---|
| H | 5.0 × 109 * |
| H5 | 4.2 × 109 |
| H9 | 2.7 × 109 |
| G | 1.7 × 107 * |
3.4. Peptide Nanogel Accumulation in Pancreatic Cancer Cells
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv. Healthc. Mater. 2020, 9, 1901223. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Islam, R.; Islam, W.; Yin, H.; Subr, V.; Maeda, H. Augmentation of EPR effect and efficacy of anticancer nanomedicine by carbon monoxide generating agents. Pharmaceutics 2019, 11, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasery, M.M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin delivery mediated by bio-based nanoparticles: A review. Molecules 2020, 25, 689. [Google Scholar]
- Sun, M.; Lee, J.; Chen, Y.; Hoshino, K. Studies of nanoparticle delivery with in vitro bio-engineered microtissues. Bioact. Mater. 2020, 5, 924–937. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Andrade, R.G.D.; Ribeiro, B.C.; Fernandes, A.V.F.; Rodrigues, A.R.O.; Martins, J.A.; Ferreira, P.M.T.; Coutinho, P.J.G.; Castanheira, E.M.S. Magnetoliposomes incorporated in peptide-based hydrogels: Towards development of magnetolipogels. Nanomaterials 2020, 10, 1702. [Google Scholar] [CrossRef]
- Martínez-Jothar, L.; Barendrecht, A.D.; de Graaff, A.M.; Oliveira, S.; van Nostrum, C.F.; Schiffelers, R.M.; Hennink, W.E.; Fens, M.H.A.M. Endothelial cell targeting by cRGD-functionalized polymeric nanoparticles under static and flow conditions. Nanomaterials 2020, 10, 1353. [Google Scholar] [CrossRef]
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of nanoparticles in biomedical imaging. Nanoscale 2019, 11, 799–819. [Google Scholar] [CrossRef]
- Yuba, E.; Fukaya, Y.; Yanagihara, S.; Kasho, N.; Harada, A. Development of mannose-modified carboxylated curdlan-coated liposomes for antigen presenting cell targeted antigen delivery. Pharmaceutics 2020, 12, 754. [Google Scholar] [CrossRef]
- Slapak, E.J.; Mandili, M.; Bijlsma, M.F.; Spek, C.A. Mesoporous silica nanoparticle-based drug delivery systems for the treatment of pancreatic cancer: A systematic literature overview. Pharmaceutics 2022, 14, 390. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.C.; Reis, D.P.; Pereira, M.C.; Coelho, M.A.N. Gold nanoparticles for targeting varlitinib to human pancreatic cancer cells. Pharmaceutics 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, R.W.J.; Wilson, O.M.; Crooks, R.M. Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles. J. Phys. Chem. B 2005, 109, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Anton, N.; Benoit, J.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates—A review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Ono, M.; Eiji, Y.; Harada, A. In vitro sonodynamic therapeutic effect of poly ion complex micelles incorporating titanium dioxide nanoparticles. Nanomaterials 2017, 7, 268. [Google Scholar] [CrossRef] [Green Version]
- Yasunaga, M.; Manabe, S.; Matsumura, Y. New concept of cytotoxic immunoconjugate therapy targeting cancer induced fibrin clots. Cancer Sci. 2011, 102, 1396–1402. [Google Scholar] [CrossRef]
- Manzur, A.; Oluwasanmi, A.; Moss, D.; Curtis, A.; Hoskins, C. Nanotechnologies in pancreatic cancer therapy. Pharmaceutics 2017, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Alhakamy, N.A.; Ahmed, A.A.; Fahmy, U.A.; Md, S. Apamin-conjugated alendronate sodium nanocomplex for management of pancreatic cancer. Pharmaceutics 2021, 14, 729. [Google Scholar] [CrossRef]
- Kimura, A.; Jo, J.; Yoshida, F.; Hong, Z.; Tabata, Y.; Sumiyoshi, A.; Taguchi, M.; Aoki, I. Ultra-small size gelatin nanogel as a blood brain barrier impermeable contrast agent for magnetic resonance imaging. Acta Biomater. 2021, 125, 290–299. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Ulanski, P.; Pajewski, L.A.; Yoshii, F.; Makuuchi, K. Radiation formation of hydrogels for biomedical purposes. Some remarks and comments. Radiat. Phys. Chem. 1995, 46, 161–168. [Google Scholar] [CrossRef]
- Kimura, A.; Yoshida, F.; Ueno, M.; Taguchi, M. Application of radiation crosslinking technique to development of gelatin scaffold for tissue engineering. Radiat. Phys. Chem. 2021, 180, 109287. [Google Scholar] [CrossRef]
- Garrison, W.M. Reaction mechanisms in the radiolysis of peptides, polypeptides, and proteins. Chem. Rev. 1987, 87, 381–398. [Google Scholar] [CrossRef] [Green Version]
- Kimura, A.; Ueno, M.; Arai, T.; Oyama, K.; Taguchi, M. Radiation crosslinked smart peptide nanoparticles: A new platform for tumor imaging. Nanomaterials 2021, 11, 714. [Google Scholar] [CrossRef]
- Saadat, M.; Zahednezhad, F.; Zakeri-Milani, P.; Heidari, H.R.; Shahbazi-Mojarrad, J.; Valizadeh, H. Drug targeting strategies based on charge dependent uptake of nanoparticles into cancer cells. J. Pharm. Pharm. Sci. 2019, 22, 131–364. [Google Scholar] [CrossRef] [Green Version]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumors depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Yue, Z.G.; Wei, W.; Lv, P.P.; Yue, H.; Wang, L.Y.; Su, Z.G.; Ma, G.H. Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Perez, J.M. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano 2010, 4, 5321–5331. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Dunshee, L.C.; Sullivan, M.O.; Kiick, K.L. Manipulation of the dually thermoresponsive behavior of peptide-based vesicles through modification of collagen-like peptide domains. Bioeng. Transl. Med. 2019, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Galano, A.; Torresb, A.C. OH radical reactions with phenylalanine in free and peptide forms. Org. Biomol. Chem. 2008, 6, 732–738. [Google Scholar] [CrossRef]
- Pauling, L.; Corey, R.B.; Branson, H.R. The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Natl. Acad. Sci. USA 1951, 37, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R.J. Tables of bond lengths determined by x-ray and neutron diffraction. Part I. bond lengths in organic compounds. Chem. Soc. Perkin Trans. 1987, 2, S1. [Google Scholar] [CrossRef]
- Buxton, G.V.; Stuart, C.R. Re-evaluation of the thiocyanate dosimeter for pulse radiolysis. J. Chem. Soc. Faraday Trans. 1995, 91, 279–281. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O–) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Klassen, N.V. Radiation Chemistry: Principles and Applications; VCH: New York, NY, USA, 1987. [Google Scholar]
- Schuler, R.H.; Patterson, L.K.; Janata, E. Yield for the scavenging of OH radicals in the radiolysis of N2O-saturated aqueous solutions. J. Phys. Chem. 1980, 84, 2088–2089. [Google Scholar] [CrossRef]
- Rao, P.S.; Simic, M.; Hayon, E. Pulse radiolysis study of imidazole and histidine in water. J. Phys. Chem. 1975, 79, 1260–1263. [Google Scholar] [CrossRef]
- Masuda, T.; Nakano, S.; Kondo, M. Rate constants for the reactions of OH radicals with the enzyme proteins as determined by the p-nitrosodimethylaniline method. J. Radiat. Res. 1973, 14, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Michon, T.; Chenu, M.; Kellershon, N.; Desmadril, M.; Horseradish, J.G. Peroxidase oxidation of tyrosine-containing peptides and their subsequent polymerization: A kinetic study. Biochemistry 1997, 36, 8504–8513. [Google Scholar] [CrossRef]
- Canals, F. Signal transmission by epidermal growth factor receptor: Coincidence of activation and dimerization. Biochemistry 1992, 31, 4493–4501. [Google Scholar] [CrossRef]
- Cescon, L.A.; Coraor, G.R.; Dessauer, R.; Silversmith, E.F.; Urban, E.J. Some properties of triarylimidazolyl radicals and their dimers. J. Org. Chem. 1971, 36, 2262–2267. [Google Scholar]
- Chen, X.; Schuler, R.H. Directing effects of phenyl substitution in the reaction of OH radical with aromatics: The radiolytic hydroxylation of biphenyl. J. Phys. Chem. 1993, 97, 421–425. [Google Scholar] [CrossRef]
- Sehested, K.; Hart, E.J. Formation and decay of the biphenyl cation radical in aqueous acidic solution. J. Phys. Chem. 1975, 79, 1639–1642. [Google Scholar] [CrossRef]
- Felber, T.; Schaefer, T.; Herrmann, H. OH-Initiated oxidation of imidazoles in tropospheric aqueous phase chemistry. J. Phys. Chem. A. 2019, 123, 1505–1513. [Google Scholar] [CrossRef]
- Mvula, E.; Schuchmann, M.N.; Sonntag, C. Reactions of phenol-OH-adduct radicals. Phenoxyl radical formation by water elimination vs. oxidation by dioxygen. J. Chem. Soc. Perkin Trans. 2001, 2, 264–268. [Google Scholar] [CrossRef]
- Hashimoto, S.; Miyata, T.; Washino, M.; Kawakami, W. A liquid chromatographic study on the radiolysis of phenol in aqueous solution. Environ. Sci. Technol. 1979, 13, 71–75. [Google Scholar] [CrossRef]
- Pan, X.M.; Schuchmann, M.N.; Sonntag, C. Oxidation of benzene by the OH radical. A product and pulse radiolysis study in oxygenated aqueous solution. J. Chem. Soc. Perkin Trans. 1993, 2, 289–297. [Google Scholar] [CrossRef]
- Gao, D.; Vela, I.; Sboner, A.; Iaquinta, P.J.; Karthaus, W.R.; Gopalan, A.; Dowling, C.; Wanjala, J.N.; Undvall, E.A.; Arora, V.K.; et al. Organoid Cultures Derived from Patients with Advanced Prostate Cancer. Cell 2014, 159, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Tsai, S.; McOlash, L.; Palen, K.; Johnson, B.; Duris, C.; Yang, Q.; Dwinell, M.B.; Hunt, B.; Evans, D.B.; Gershan, J.; et al. Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer 2018, 18, 335. [Google Scholar] [CrossRef]
- Driehuis, E.; Hoeck, A.V.; Moore, K.; Clevers, H. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc. Natl. Acad. Sci. USA 2019, 116, 26580–26590. [Google Scholar] [CrossRef]









| Abbreviation | Peptide Sequence |
|---|---|
| HGHGH (H5) | His-Gly-His-Gly-His |
| HGGGHGGGH (H9) | His-Gly-Gly-Gly-His-Gly-Gly-Gly-His |
| HGGGGGHGGGGGH (H13) | His-Gly-Gly-Gly-Gly-Gly-His-Gly-Gly-Gly-Gly-Gly-His |
| Peptide | log P |
|---|---|
| HGHGH (H5) | −8.07 |
| HGGGHGGGH (H9) | −12.67 |
| HGGGGGHGGGGGH (H13) | −17.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, A.; Arai, T.; Ueno, M.; Oyama, K.; Yu, H.; Yamashita, S.; Otome, Y.; Taguchi, M. Synthesis of Small Peptide Nanogels Using Radiation Crosslinking as a Platform for Nano-Imaging Agents for Pancreatic Cancer Diagnosis. Pharmaceutics 2022, 14, 2400. https://doi.org/10.3390/pharmaceutics14112400
Kimura A, Arai T, Ueno M, Oyama K, Yu H, Yamashita S, Otome Y, Taguchi M. Synthesis of Small Peptide Nanogels Using Radiation Crosslinking as a Platform for Nano-Imaging Agents for Pancreatic Cancer Diagnosis. Pharmaceutics. 2022; 14(11):2400. https://doi.org/10.3390/pharmaceutics14112400
Chicago/Turabian StyleKimura, Atsushi, Tadashi Arai, Miho Ueno, Kotaro Oyama, Hao Yu, Shinichi Yamashita, Yudai Otome, and Mitsumasa Taguchi. 2022. "Synthesis of Small Peptide Nanogels Using Radiation Crosslinking as a Platform for Nano-Imaging Agents for Pancreatic Cancer Diagnosis" Pharmaceutics 14, no. 11: 2400. https://doi.org/10.3390/pharmaceutics14112400