Structure, Properties, and Release Kinetics of the Polymer/Insect Repellent System Poly (l-Lactic Acid)/Ethyl Butylacetylaminopropionate (PLLA/IR3535)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.2. Instrumentation
3. Results and Discussion
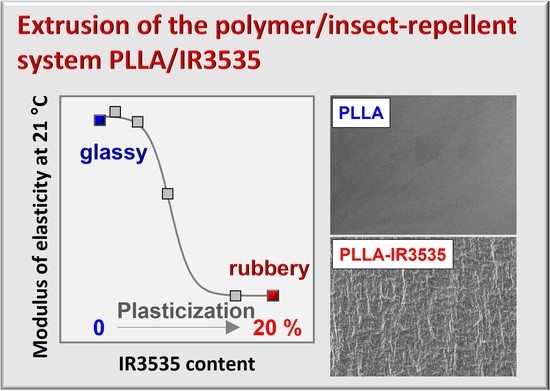
3.1. Repellent Content of Extrudates
3.2. Morphology and Fracture Behavior of Extrudates
3.3. DSC Analysis of Extrudates
3.4. Thermal Stability of Extrudates
3.5. WAXS and SAXS Analysis
3.6. POM Analysis of Annealed PLLA/IR3535 Extrudates
3.7. Mechanical Properties of PLLA/IR3535 Extrudates
3.8. Repellent Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric biomaterials for medical implants and devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.L.; Burdick, J.A. Introduction: Polymeric biomaterials. Chem. Rev. 2021, 121, 10789–10791. [Google Scholar] [CrossRef]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef] [PubMed]
- Calori, I.R.; Braga, G.; de Jesus, P.d.C.C.; Bi, H.; Tedesco, A.C. Polymer scaffolds as drug delivery systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Shamsuddin, I.M.; Jafar, J.A.; Shawai, A.S.A.; Yusuf, S.; Lateefah, M.; Aminu, I. Bioplastics as better alternative to petroplastics and their role in national sustainability: A review. Adv. Biosci. Bioeng. 2017, 5, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Srivasatva, A.K.; Singh, A.; Singh, P.; Verma, S.; Vats, M.; Sagadevan, S. Biopolymers as renewable polymeric materials for sustainable development—An overview. Polimery 2022, 67, 185–196. [Google Scholar] [CrossRef]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef] [Green Version]
- Faizan, M.; Nadeem, H.; Arif, A.; Zaheer, W. Bioplastics from biopolymers: An eco-friendly and sustainable solution of plastic pollution. Polym. Sci. Ser. C 2021, 63, 47–63. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.B.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R. Synthesis, Structure and Properties of Poly (Lactic Acid); Springer: Cham, Switzerland, 2018; Volume 279. [Google Scholar]
- Di Lorenzo, M.L.; Androsch, R. Industrial Applications of Poly(Lactic Acid); Springer: Cham, Switzerland, 2018; Volume 282. [Google Scholar]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-l-lactic acid (PLLA)-based biomaterials for regenerative medicine: A review on processing and applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef] [PubMed]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-lactic acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Martin, O.; Averous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, Y.S.; Topolkaraev, V.; Hiltner, A.; Baer, E. Aging of poly(lactide)/poly(ethylene glycol) blends. Part 2. Poly(lactide) with high stereoregularity. Polymer 2003, 44, 5711–5720. [Google Scholar] [CrossRef]
- Kulinski, Z.; Piorkowska, E.; Gadzinowska, K.; Stasiak, M. Plasticization of poly(l-lactide) with poly(propylene glycol). Biomacromolecules 2006, 7, 2128–2135. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Effect of nucleation and plasticization on the crystallization of poly(lactic acid). Polymer 2007, 48, 6855–6866. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.; Yang, F.; Hao, Y.; Wu, G.; Zhang, H.; Dong, L. Thermal, mechanical, and rheological properties of plasticized poly(l-lactic acid). J. Appl. Polym. Sci. 2013, 127, 2832–2839. [Google Scholar] [CrossRef]
- Avolio, R.; Castaldo, R.; Gentile, G.; Ambrogi, V.; Fiori, S.; Avella, M.; Cocca, M.; Errico, M.E. Plasticization of poly(lactic acid) through blending with oligomers of lactic acid: Effect of the physical aging on properties. Eur. Polym. J. 2015, 66, 533–542. [Google Scholar] [CrossRef]
- Dobrzynska-Mizera, M.; Knitter, M.; Mallardo, S.; Del Barone, M.C.; Santagata, G.; Di Lorenzo, M.L. Thermal and thermo-mechanical properties of poly(L-lactic Acid) biocomposites containing beta-cyclodextrin/d-limonene inclusion complex. Materials 2021, 14, 2569. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.L.; Longo, A. N,N-Diethyl-3-methylbenzamide (DEET): A mosquito repellent as functional plasticizer for poly(l -lactic acid). Thermochim. Acta 2019, 677, 180–185. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Panariello, L.; Gigante, V.; Coltelli, M.B.; Lazzeri, A. Sustainable micro and nano additives for controlling the migration of a biobased plasticizer from pla-based flexible films. Polymers 2020, 12, 1336. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, J.D.; Strickman, D.; Zwiebel, L.J. The future of insect repellent discovery and development. Outlooks Pest Manag. 2014, 25, 265–270. [Google Scholar] [CrossRef]
- Tavares, M.; da Silva, M.R.M.; de Oliveira de Siqueira, L.B.; Rodrigues, R.A.S.; Bodjolle-d’Almeida, L.; dos Santos, E.P.; Ricci-Júnior, E. Trends in insect repellent formulations: A review. Int. J. Pharm. 2018, 539, 190–209. [Google Scholar] [CrossRef]
- Sungkapreecha, C.; Iqbal, N.; Gohn, A.M.; Focke, W.W.; Androsch, R. Phase behavior of the polymer/drug system PLA/DEET. Polymer 2017, 126, 116–125. [Google Scholar] [CrossRef]
- Sungkapreecha, C.; Beily, M.J.; Kressler, J.; Focke, W.W.; Androsch, R. Phase behavior of the polymer/drug system PLA/DEET: Effect of PLA molar mass on subambient liquid-liquid phase separation. Thermochim. Acta 2018, 660, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Sungkapreecha, C.; Iqbal, N.; Focke, W.W.; Androsch, R. Crystallization of poly (l-lactic acid) in solution with the mosquito-repellent N, N-diethyl-3-methylbenzamide. Polym. Cryst. 2019, 2, e10029. [Google Scholar] [CrossRef]
- Sungkapreecha, C.; Focke, W.W.; Androsch, R. Competition between liquid-liquid de-mixing, crystallization, and glass transition in solutions of PLA of different stereochemistry and DEET. Chin. J. Polym. Sci. 2020, 38, 174–178. [Google Scholar] [CrossRef]
- Bonadies, I.; Longo, A.; Androsch, R.; Jehnichen, D.; Göbel, M.; Di Lorenzo, M.L. Biodegradable electrospun PLLA fibers containing the mosquito-repellent DEET. Eur. Polym. J. 2019, 113, 377–384. [Google Scholar] [CrossRef]
- Ferreira, I.; Brünig, H.; Focke, W.; Boldt, R.; Androsch, R.; Leuteritz, A. Melt-spun poly (d,l-lactic acid) monofilaments containing N, N-diethyl-3-methylbenzamide as mosquito repellent. Materials 2021, 14, 638. [Google Scholar] [CrossRef] [PubMed]
- Izadi, H.; Focke, W.W.; Asaadi, E.; Maharaj, R.; Pretorius, J.; Loots, M.T. A promising azeotrope-like mosquito repellent blend. Sci. Rep. 2017, 7, 10273. [Google Scholar] [CrossRef]
- Mapossa, A.B.; Sitoe, A.; Focke, W.W.; Izadi, H.; Du Toit, E.L.; Androsch, R.; Sungkapreecha, C.; Van Der Merwe, E.M. Mosquito repellent thermal stability, permeability and air volatility. Pest Manag. Sci. 2020, 76, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Butylacetylaminopropionate, E. WHO Specifications and Evaluations for Public Health Pesticides. Available online: https://archive.epa.gov/osa/hsrb/web/pdf/whoir3535evaluationapril2006.pdf (accessed on 16 September 2022).
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2022.
- WHO Guidelines for Malaria. Available online: WHO-UCN-GMP-2022.01-Rev.2-eng.pdf (accessed on 16 September 2022).
- Du, F.; Schick, C.; Androsch, R. Full-composition-range glass transition behavior of the polymer/solvent system poly (lactic acid)/ethyl butylacetylaminopropionate (PLA/IR3535®). Polymer 2020, 209, 123058. [Google Scholar] [CrossRef]
- Du, F.; Yener, H.E.; Hillrichs, G.; Boldt, R.; Androsch, R. Crystallization-induced polymer scaffold formation in the polymer/drug delivery system poly(l-lactic acid)/ethyl butylacetylaminopropionate (PLLA/IR3535). Biomacromolecules 2021, 22, 3950–3959. [Google Scholar] [CrossRef] [PubMed]
- Mapossa, A.B.; López-Beceiro, J.; Díaz-Díaz, A.M.; Artiaga, R.; Moyo, D.S.; Mphateng, T.N.; Focke, W.W. Properties of mosquito repellent-plasticized poly(lactic acid) strands. Molecules 2021, 26, 5890. [Google Scholar] [CrossRef]
- Lloyd, D.R. Microporous membrane formation via thermally induced phase separation. I. Solid-liquid phase separation. J. Membr. Sci. 1990, 50, 239–261. [Google Scholar] [CrossRef]
- Kim, S.S.; Lloyd, D.R. Thermodynamics of polymer/diluent systems for thermally induced phase separation: 3. Liquid-liquid phase separation systems. Polymer 1992, 33, 1047–1057. [Google Scholar] [CrossRef]
- Du, F.; Rupp, H.; Jariyavidyanont, K.; Janke, A.; Petzold, A.; Binder, W.; Androsch, R. 3D-Printing of the polymer/insect-repellent system poly(l-lactic acid)/ethyl butylacetylaminopropionate (PLLA/IR3535). Int. J. Pharm. 2022, 624, 122023. [Google Scholar] [CrossRef]
- Product information, TotalEnergies Corbion Ltd. 2021. Available online: https://www.totalenergies-corbion.com/media/w5cgez0x/pds-luminy-l175-rmb20.pdf (accessed on 11 July 2022).
- Carbolution Chemicals GmbH, Ethyl butylacetylaminopropionate Product Information. Available online: https://www.carbolution.de/product_info.php?products_id=3221 (accessed on 16 September 2022).
- DIN EN ISO 527-2:2012-06; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion plastics (ISO 527-2:2012); German Version EN ISO 527-2:2012. 2012. Available online: https://www.beuth.de/en/standard/din-en-iso-527-2/148232494 (accessed on 7 August 2022).
- Price, D.M.; Hourston, D.J.; Dumont, F. Thermogravimetry of polymers. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; Wiley: Chichester, UK, 2000; pp. 8094–8105. [Google Scholar]
- Saadatkhah, N.; Carillo Garcia, A.; Ackermann, S.; Leclerc, P.; Latifi, M.; Samih, S.; Patience, G.S.; Chaouki, J. Experimental methods in chemical engineering: Thermogravimetric analysis—TGA. Can. J. Chem. Eng. 2020, 98, 34–43. [Google Scholar] [CrossRef]
- Gerasimov, D.N.; Yurin, E.I. Kinetics of Evaporation; Springer: Cham, Switzerland, 2018; Volume 68. [Google Scholar]
- Sakurada, I.; Nakajima, A.; Fujiwara, H. Vapor pressures of polymer solutions. II. Vapor pressure of the poly (vinyl alcohol)-water system. J. Polym. Sci. 1959, 35, 497–505. [Google Scholar] [CrossRef]
- Wei, X.F.; Linde, E.; Hedenqvist, M.S. Plasticiser loss from plastic or rubber products through diffusion and evaporation. NPJ Mater. Degrad. 2019, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- McNeill, I.C.; Leiper, H.A. Degradation studies of some polyesters and polycarbonates—1. Polylactide: General features of the degradation under programmed heating conditions. Polym. Degr. Stab. 1985, 11, 267–285. [Google Scholar] [CrossRef]
- Kopinke, F.D.; Remmler, M.; Mackenzie, K.; Möder, M.; Wachsen, O. Thermal decomposition of biodegradable polyesters—II. Poly (lactic acid). Polym. Degr. Stab. 1996, 53, 329–342. [Google Scholar] [CrossRef]
- Androsch, R.; Di Lorenzo, M.L.; Schick, C. Optical microscopy to study crystal nucleation in polymers using a fast scanning chip calorimeter for precise control of the nucleation pathway. Macromol. Chem. Phys. 2018, 219, 1700479. [Google Scholar] [CrossRef]
- Schick, C.; Androsch, R. Nucleation-controlled semicrystalline morphology of bulk polymers. Polym. Cryst. 2018, 1, e10036. [Google Scholar] [CrossRef]
- Miyata, T.; Masuko, T. Crystallization behaviour of poly (l-lactide). Polymer 1998, 39, 5515–5521. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Tsubakihara, S.; Iura, K.; Ono, Y.; Dan, Y.; Takahashi, K. Crystallization behavior of poly (l-lactic acid). Polymer 2006, 47, 7554–7563. [Google Scholar] [CrossRef]
- Giles, H.F., Jr.; Mount, E.M., III; Wagner, J.R., Jr. Extrusion, The Definitive Processing Guide and Handbook; William Andrew Inc.: Norwich, UK, 2005. [Google Scholar]
- Sitoe, A.; Mapossa, A.B.; Focke, W.W.; Muiambo, H.; Androsch, R.; Wesley-Smith, J. Development, characterization and modeling of mosquito repellent release from microporous devices. SPE Polym. 2020, 1, 90–100. [Google Scholar] [CrossRef]
- Mapossa, A.B.; Sibanda, M.M.; Sitoe, A.; Focke, W.W.; Braack, L.; Ndonyane, C.; Mouatcho, J.; Smart, J.; Muaimbo, H.; Androsch, R.; et al. Microporous polyolefin strands as controlled-release devices for mosquito repellents. Chem. Eng. J. 2019, 360, 435–444. [Google Scholar] [CrossRef]
- Yener, H.E.; Erdmann, R.; Jariyavidyanont, K.; Mapossa, A.B.; Focke, W.W.; Hillrichs, G.; Androsch, R. Slow-DEET-release mosquito-repellent system based on poly(butylene succinate). ACS Omega 2022, 7, 8377–8384. [Google Scholar] [CrossRef]
- Pyda, M.; Bopp, R.C.; Wunderlich, B. Heat capacity of poly (lactic acid). J. Chem. Thermodyn. 2004, 36, 731–742. [Google Scholar] [CrossRef]
- Mano, J.F.; Ribelles, J.G.; Alves, N.M.; Sanchez, M.S. Glass transition dynamics and structural relaxation of PLLA studied by DSC: Influence of crystallinity. Polymer 2005, 46, 8258–8265. [Google Scholar] [CrossRef] [Green Version]
- Pan, P.; Zhu, B.; Inoue, Y. Enthalpy relaxation and embrittlement of poly (l-lactide) during physical aging. Macromolecules 2007, 40, 9664–9671. [Google Scholar] [CrossRef]
- Naeem Iqbal, H.M.; Sungkapreecha, C.; Androsch, R. Enthalpy relaxation of the glass of poly (l-lactic acid) of different d-isomer content and its effect on mechanical properties. Polym. Bull. 2017, 74, 2565–2573. [Google Scholar] [CrossRef]
- Pan, P.; Zhu, B.; Kai, W.; Dong, T.; Inoue, Y. Effect of crystallization temperature on crystal modifications and crystallization kinetics of poly (l-lactide). J. Appl. Polym. Sci. 2008, 107, 54–62. [Google Scholar] [CrossRef]
- Pan, P.; Inoue, Y. Polymorphism and isomorphism in biodegradable polyesters. Prog. Polym. Sci. 2009, 34, 605–640. [Google Scholar] [CrossRef]
- Kawai, T.; Rahman, N.; Matsuba, G.; Nishida, K.; Kanaya, T.; Nakano, M.; Okamoto, H.; Kawada, J.; Usuki, A.; Honma, N.; et al. Crystallization and melting behavior of poly(l-lactic acid). Macromolecules 2007, 40, 9463–9469. [Google Scholar] [CrossRef]
- Wasanasuk, K.; Tashiro, K.; Hanesaka, M.; Ohhara, T.; Kurihara, K.; Kuroki, R.; Tamada, T.; Ozeki, T.; Kanamoto, T. Crystal structure analysis of poly (l-lactic acid) α form on the basis of the 2-dimensional wide-angle synchrotron X-ray and neutron diffraction measurements. Macromolecules 2011, 44, 6441–6452. [Google Scholar] [CrossRef]
- Kolstad, J.J. Crystallization kinetics of poly (l-lactide-co-meso-lactide). J. Appl. Polym. Sci. 1996, 62, 1079–1091. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Rubino, P.; Luijkx, R.; Hélou, M. Influence of chain structure on crystal polymorphism of poly (lactic acid). Part 1: Effect of optical purity of the monomer. Colloid Polym. Sci. 2014, 292, 399–409. [Google Scholar] [CrossRef]
- Salmerón Sánchez, M.; Mathot, V.B.F.; Vanden Poel, G.; Gómez Ribelles, J.L. Effect of the cooling rate on the nucleation kinetics of poly (l-lactic acid) and its influence on morphology. Macromolecules 2007, 40, 7989–7997. [Google Scholar] [CrossRef]
- Androsch, R.; Iqbal, H.M.N.; Schick, C. Non-isothermal crystal nucleation of poly (l-lactic acid). Polymer 2015, 81, 151–158. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C.; Di Lorenzo, M.L. Kinetics of nucleation and growth of crystals of poly (l-lactic acid). Adv. Polym. Sci. 2017, 279, 235–272. [Google Scholar]
- Flory, P.J. Thermodynamics of crystallization in high polymers. IV. A Theory of crystalline states and fusion in polymers, copolymers, and their mixtures with diluents. J. Chem. Phys. 1949, 17, 223–240. [Google Scholar] [CrossRef]
- Rim, P.B.; Runt, J.P. Melting point depression in crystalline/compatible polymer blends. Macromolecules 1984, 17, 1520–1526. [Google Scholar] [CrossRef]
- Burghardt, W.R. Phase diagrams for binary polymer systems exhibiting both crystallization and limited liquid-liquid miscibility. Macromolecules 1989, 22, 2482–2486. [Google Scholar] [CrossRef]
- Xiao, H.; Lu, W.; Yeh, J.T. Effect of plasticizer on the crystallization behavior of poly (lactic acid). J. Appl. Polym. Sci. 2009, 113, 112–121. [Google Scholar] [CrossRef]
- Paul, D.R.; Barlow, J.W. Crystallization from miscible polymer blends. In Polymer Alloys II; Springer: Boston, MA, USA, 1980; pp. 239–253. [Google Scholar]
- Ali, F.; Chang, Y.W.; Kang, S.C.; Yoon, J.Y. Thermal, mechanical and rheological properties of poly (lactic acid)/epoxidized soybean oil blends. Polym. Bull. 2009, 62, 91–98. [Google Scholar] [CrossRef]
- Schick, C.; Donth, E. Characteristic length of glass transition: Experimental evidence. Phys. Scripta 1991, 43, 423–429. [Google Scholar] [CrossRef]
- Arndt, M.; Stannarius, R.; Groothues, H.; Hempel, E.; Kremer, F. Length scale of cooperativity in the dynamic glass transition. Phys. Rev. Lett. 1997, 79, 2077–2080. [Google Scholar] [CrossRef]
- Jariyavidyanont, K.; Du, M.; Yu, Q.; Thurn-Albrecht, T.; Schick, C.; Androsch, R. Bulk Enthalpy of melting of poly (l-lactic acid) (PLLA) determined by fast scanning chip calorimetry. Macromol. Rap. Commun. 2022, 43, 2200148. [Google Scholar] [CrossRef]
- Jariyavidyanont, K.; Schick, C.; Androsch, R. The bulk enthalpy of melting of α’-crystals of poly (l-lactic acid) determined by fast scanning chip calorimetry. Thermochim. Acta 2022, 717, 179349. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, Y.; Sato, H.; Tsuji, H.; Noda, I.; Yan, S.; Ozaki, Y. Crystal modifications and thermal behavior of poly (l-lactic acid) revealed by infrared spectroscopy. Macromolecules 2005, 38, 8012–8021. [Google Scholar] [CrossRef]
- Meaurio, E.; López-Rodríguez, N.; Sarasua, J.R. Infrared spectrum of poly (l-lactide): Application to crystallinity studies. Macromolecules 2006, 39, 9291–9301. [Google Scholar] [CrossRef]
- Chen, X.; Han, L.; Zhang, T.; Zhang, J. Influence of crystal polymorphism on crystallinity calculation of poly (l-lactic acid) by infrared spectroscopy. Vibr. Spectr. 2014, 70, 1–5. [Google Scholar] [CrossRef]
- Du, M.; Jariyavidyanont, K.; Boldt, R.; Tariq, M.; Fischer, M.; Spoerer, Y.; Kuehnert, I.; Androsch, R. Crystal-nuclei formation during injection-molding of poly (l-lactic acid). Polymer 2022, 250, 124897. [Google Scholar] [CrossRef]
- Pan, P.; Kai, W.; Zhu, B.; Dong, T.; Inoue, Y. Polymorphous crystallization and multiple melting behavior of poly (l-lactide): Molecular weight dependence. Macromolecules 2007, 40, 6898–6905. [Google Scholar] [CrossRef]
- Cocca, M.; Di Lorenzo, M.L.; Malinconico, M.; Frezza, V. Influence of crystal polymorphism on mechanical and barrier properties of poly (l-lactic acid). Eur. Polym. J. 2011, 47, 1073–1080. [Google Scholar] [CrossRef]
- Miyata, T.; Masuko, T. Morphology of poly(l-lactide) solution-grown crystals. Polymer 1997, 38, 4003–4009. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C.; Di Lorenzo, M.L. Melting of conformationally disordered crystals (α′-phase) of poly (l-lactic acid). Macromol. Chem. Phys. 2014, 215, 1134–1139. [Google Scholar] [CrossRef]
- Schulz, M.; Seidlitz, A.; Kurz, R.; Bärenwald, R.; Petzold, A.; Saalwächter, K.; Thurn-Albrecht, T. The underestimated effect of intracrystalline chain dynamics on the morphology and stability of semicrystalline polymers. Macromolecules 2018, 51, 8377–8385. [Google Scholar] [CrossRef]
- Seidlitz, A.; Thurn-Albrecht, T. Chapter 9. Small-angle X-ray scattering for morphological analysis of semicrystalline polymers. Polym. Morphol. Princ. Charact. Process. 2016, 15, 151–164. [Google Scholar]
- Runt, J.; Kanchanasopa, M. Crystallinity Determination. In Encyclopedia of Polymer Science and Technology; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Ryan, A.J.; Bras, W.; Mant, G.R.; Derbyshire, G.E. A direct method to determine the degree of crystallinity and lamellar thickness of polymers: Application to polyethylene. Polymer 1994, 35, 4537–4544. [Google Scholar] [CrossRef]
- Toda, A.; Androsch, R.; Schick, C. Insights into polymer crystallization and melting from fast scanning chip calorimetry. Polymer 2016, 91, 239–263. [Google Scholar] [CrossRef]
- Schick, C.; Androsch, R.; Schmelzer, J.W.P. Homogeneous crystal nucleation in polymers. J. Phys. Cond. Matter 2017, 29, 453002. [Google Scholar] [CrossRef]
- Cristea, M.; Ionita, D.; Iftime, M.M. Dynamic mechanical analysis investigations of PLA-based renewable materials: How are they useful? Materials 2020, 13, 5302. [Google Scholar] [CrossRef]
- Perego, G.; Cella, G.D.; Bastioli, C. Effect of molecular weight and crystallinity on poly (lactic acid) mechanical properties. J. Appl. Polym. Sci. 1996, 59, 37–43. [Google Scholar] [CrossRef]
- Mathot, V.; Pyda, M.; Pijpers, T.; Poel, G.V.; Van de Kerkhof, E.; Van Herwaarden, S.; Van Herwaarden, F.; Leenaers, A. The Flash DSC 1, a power compensation twin-type, chip-based fast scanning calorimeter (FSC): First findings on polymers. Thermochim. Acta 2011, 522, 36–45. [Google Scholar] [CrossRef]
- Kolesov, I.; Mileva, D.; Androsch, R.; Schick, C. Structure formation of polyamide 6 from the glassy state by fast scanning chip calorimetry. Polymer 2011, 52, 5156–5165. [Google Scholar] [CrossRef]
- Crist, B.; Fisher, C.J.; Howard, P.R. Mechanical properties of model polyethylenes: Tensile elastic modulus and yield stress. Macromolecules 1989, 22, 1709–1718. [Google Scholar] [CrossRef]
- Zia, Q.; Radusch, H.J.; Androsch, R. Deformation behavior of isotactic polypropylene crystallized via a mesophase. Polym. Bull. 2009, 63, 755–771. [Google Scholar] [CrossRef]
- Youssef, G. Applied Mechanics of Polymers: Properties, Processing, and Behavior; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Ryan, J.J.; Casalini, R.; Orlicki, J.A.; Lundin, J.G. Controlled release of the insect repellent picaridin from electrospun nylon-6,6 nanofibers. Polym. Adv. Technol. 2020, 31, 3039–3047. [Google Scholar] [CrossRef]












| PLLA/IR3535 Ratio (m%) | Screw Speed (1/min) | Main Feeder (1/min) | Engine Load (%) | Die Pressure (bar) | Melt Temperature (°C) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Die | Zone 2 | Zone 4 | Zone 6 | Zone 8 | |||||
| 100/0 | 250 | 17.0 | 61 | 10 | 190 | 186 | 187 | 191 | 199 |
| 98/2 | 250 | 35.8 | 88 | 21 | 189 | 186 | 184 | 191 | 199 |
| 95/5 | 250 | 20.8 | 58 | 12 | 190 | 184 | 187 | 190 | 199 |
| 90/10 | 250 | 9.8 | 38 | 5 | 186 | 182 | 185 | 189 | 198 |
| 80/20 | 250 | 13.1 | 36 | 5 | 188 | 182 | 184 | 187 | 197 |
| 70/30 1 | 250 | 7.6 | 10 | 1 | 187 | 182 | 184 | 187 | 197 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, F.; Erdmann, R.; Petzold, A.; Wutzler, A.; Leuteritz, A.; Nase, M.; Androsch, R. Structure, Properties, and Release Kinetics of the Polymer/Insect Repellent System Poly (l-Lactic Acid)/Ethyl Butylacetylaminopropionate (PLLA/IR3535). Pharmaceutics 2022, 14, 2381. https://doi.org/10.3390/pharmaceutics14112381
Du F, Erdmann R, Petzold A, Wutzler A, Leuteritz A, Nase M, Androsch R. Structure, Properties, and Release Kinetics of the Polymer/Insect Repellent System Poly (l-Lactic Acid)/Ethyl Butylacetylaminopropionate (PLLA/IR3535). Pharmaceutics. 2022; 14(11):2381. https://doi.org/10.3390/pharmaceutics14112381
Chicago/Turabian StyleDu, Fanfan, Rafael Erdmann, Albrecht Petzold, Andre Wutzler, Andreas Leuteritz, Michael Nase, and René Androsch. 2022. "Structure, Properties, and Release Kinetics of the Polymer/Insect Repellent System Poly (l-Lactic Acid)/Ethyl Butylacetylaminopropionate (PLLA/IR3535)" Pharmaceutics 14, no. 11: 2381. https://doi.org/10.3390/pharmaceutics14112381