An Overview of Herbal-Based Antidiabetic Drug Delivery Systems: Focus on Lipid- and Inorganic-Based Nanoformulations
Abstract
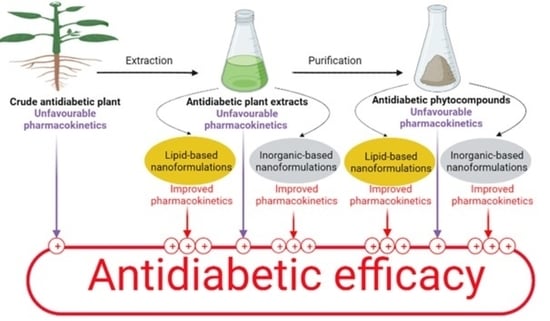
:1. Introduction
2. Diabetes Importance and Treatment
3. Herbal-Based Antidiabetic Medicines and Overview of The Antidiabetic Activity of Some Phytocompounds
3.1. Phenolic Compounds
3.1.1. Quercetin
3.1.2. Curcumin
3.1.3. Naringenin
3.1.4. Resveratrol
3.1.5. Ferulic Acid
3.1.6. Myricetin
3.1.7. Liquiritin
3.1.8. Baicalin
3.2. Nitrogenous Compounds
3.2.1. Berberine
3.2.2. Betanin
3.3. Terpenic Compounds
3.3.1. Glycyrrhizin
3.3.2. Gymnemic Acids
3.3.3. Thymoquinone
3.3.4. Lutein
3.3.5. γ-Oryzanol
3.4. Miscellaneous
3.4.1. Bitter Gourd Seed Oil and Polypeptide-k
3.4.2. Sage Oil
3.5. Limitations of Current Antidiabetic Phytocompounds
4. Current Lipid- and Inorganic-Based (Metal or Metal Oxide) Nanoformulations for Antidiabetic Herbal Products
4.1. Lipid-Based Nanoparticle Formulations Containing Antidiabetic Crude Plant Extracts or Phytocompounds
| Type of Nanoparticulate Carrier | Extract/ Phytocompound Loaded | Size of the Nanocarriers | Model of the Diabetic Animal | Effects/Overcome | References |
|---|---|---|---|---|---|
| Liposomes | Betanin | 40.06 ± 6.21 nm | Streptozotocin-induced rats | B cells protection, serum insulin levels increased, and glucose-lowering effects increased; 418.70 ± 31.38 mg/dL for diabetic rats, 390.16 ± 24.31 mg/dL for diabetic rats treated with free betanin, and 185.11 ± 27.27 mg/dL for rat treated with betanin liposomes | [193] |
| Liquiritin | 91.84 ± 1.85 nm | Streptozotocin-induced diabetic rats | 8.8-fold bioavailability increased and high hypoglycemic and antioxidant effect than free liquiritin | [194] | |
| Momordica charantia, Trigonella foenum-graecum, and Withania somnifera freeze-dried alcoholic extract (50% v/v) | 1176 ± 5.6 nm | Streptozotocin-induced diabetic rats | More than 2-fold increased efficacy: on days 7, 14, and 21 of the experiment, 500 mg/kg of encapsulated extracts lowered blood glucose 17.11%, 38.39%, and 52.11%, respectively, comparable to metformin (500 mg/kg) 21.56%, 43.92%, and 54.76%. The oral unencapsulated marketed polyformulation 1000 mg/kg decreased blood glucose levels by 12.66%, 31.29%, and 45.62%, respectively, compared to baseline | [195] | |
| Hydroalcoholic extract of Pterocarpus marsupium | ND | Alloxan-induced diabetic rats | High efficacy of the liposome formulation. A total of 50 mg/100 gm body weight per day orally for 7 days of the liposome formulation decreased blood glucose levels by 70% while the free extract decreased by 28% | [196] | |
| Phytosomes | Berberine | 165.2 ± 5.1 nm | db/db diabetic mice | 3-fold bioavailability increase than free berberine | [197] |
| Combined flavonoid-rich extract from fruits of Citrullus colocynthis (L.) Momordica balsamina and Momordica dioica | 450 nm | Streptozotocin-nicotinamide-induced diabetic rats | Sustained release of the flavonoid High antidiabetic efficacy of 100 mg/kg per day of the phytosomes than 250 mg of the free methanolic extract | [198] | |
| Niosomes | Lycopene | 202 ± 41 nm | Alloxan-induced diabetic rats | Glucose-lowering effect increased with lycopene-niosomes treatment 269 ± 13.3 to 109.6 ± 11.2 mg/dL compared to free pure lycopene 265 ± 6.8 to 136.5 ± 7.8 mg/dL | [199] |
| Alcoholic extract of Gymnema sylvestre | 229.5 nm | Alloxan-induced diabetic rats | Glucose-lowering effect increased with the noisome Significant reduction in blood glucose levels by 400 mg/kg AUC 0–2 h: 75.58 ± 1.69 compared to the nonencapsulated extract AUC 0–2 h: 80.48 ± 2.31. Gymnema sylvestre niosomes and the extract reduced the blood glucose 24.01 ± 2.00% and 15.68 ± 2.06%, respectively, compared with its initial values | [200] | |
| Solid lipid nanoparticles (SLNs) | Myricetin | 76.1 nm | Streptozotocin-nicotinamide-induced diabetic mice | Decrease in blood glucose level by myricetin-SLNs 10 mg/kg was similar to that by metformin 200 mg/kg after 4 weeks of daily oral administration | [201] |
| Nanostructured lipid carriers (NLC) | Baicalin | 92 ± 3.1 nm | Streptozotocin-induced diabetic rats | High antidiabetic efficacy compared with baicalin. Decreased fasting blood glucose level 20% more and glycosylated hemoglobine 16% more than free baicalin | [202] |
| Nanoemulsions (NEs) | Bitter gourd seed oil | 93.9 ± 2.6 nm | Alloxan-induced diabetic rats | Glucose-lowering effects increased; Antioxidant effects increased (Values not given) | [172] |
| Berberine | 30.56 ± 0.35 nm | High-fat diet and streptozocin-induced diabetic mice | Bioavailability increased by 212.02%; glucose-lowering effects increased 3-fold; regulation of liver function | [203] | |
| Sage Essential Oil | 143.2 nm | Alloxan-induced diabetic wistar rats | Glucose-lowering effects increased maximum decrease in blood glucose of 74.32% compared to free sage oil 50.45%. Regeneration of pancreatic tissue | [204] | |
| Self-nano emulsifying drug delivery system (SNEDDS) | Polypeptide-k | 31.89 nm | Streptozotocin-induced diabetic rats | Hypoglycemic effects rose >60%. Increased oxidation inhibiting effect; Hyperlipidemia preventing effects increased; Renewal of pancreatic tissue | [205] |
| Curcumin | 170 nm | Streptozotocin-induced diabetic rats | Significant decrease in tumor necrosis factor alpha (TNF-alpha) in diabetic neuropathy. ~10% more than free curcumin | [206] | |
| Resveratrol | 336 ± 11.6 nm | Streptozocin diabetic-induced albino rats | Significant hypoglycemic and hypolipidemic effects at dose 10 mg/kg comparable to two time this amount with free resveratrol | [207] |
4.1.1. Vesicular lipid nanocarriers used for antidiabetic plant extracts or phytocompounds
Liposomes
Phytosomes
Niosomes
4.1.2. Nonvesicular Lipid Nanocarriers Used for Plant Extracts or Phytocompounds
Solid Lipid Nanoparticles (SLNs)
Nanostructured Lipid Carrier (NLC)-Antidiabetic Plant Extracts or Phytocompounds
Nanoemulsions and Self-Nanoemulsifying Drug Delivery Systems (SNEDDSs)
4.2. Inorganic-Based (Metal or Metal Oxide) Nanoparticle Formulations for Antidiabetic Plant Extracts or Phytocompounds
4.2.1. Green-Synthesized Silver Nanoparticles
| Inorganic Carrier | Plant Extract Used | Study Considered | Size | Findings/Outcomes | References |
|---|---|---|---|---|---|
| Silver nanoparticles (AgNPs) | Lonicera japonica aqueous leaves extract | In vitro α-amylase, and α-glucosidase inhibitory activity | 20–60 nm | Significative potential antidiabetic activity against the two enzymes with inhibitory concentration (IC50) of 54.56 µg/mL for α-amylase and 37.86 µg/mL for α-glucosidase | [248] |
| Aqueous extract of the leaves of Calophyllum tomentosum | α-amylase, α-glucosidase, and dipeptidyl peptidase IV (DPPIV) inhibition assay | 24 nm | Strong inhibition of gastric (α-glucosidase enzyme) and incretin inhibitor (DPPIV enzyme) compared to the pancreatic one (α-amylase enzyme) | [252] | |
| Musa paradisiaca aqueous stem extract | In vivo antidiabetic potential in male albino rats of Sprague–Dawley strain | 30–60 nm | Decrease in the levels of blood glucose by 26% by a dose of 50 µg/kg, similar to that of 600 µg/kg glibenclamide (28%), a similar increase in insulin level rat receiving nanoparticles and those receiving glibenclamide and glycogen levels | [249] | |
| Eysenhardtia polystachya aqueous bark extract | in vivo antidiabetic activity assay in Zebra fish, insulin secretion assay | 10–12 nm | Suitable antidiabetic activity: 5 µg/mL decreased the glycemia by 38.1% and a decrease by 44.5% was observed by 10 µg/mL, in comparison to 51% by 5 µg/mL glibenclamide Significant increase in insulin secretion, elevation of insulin receptor expression isoforms | [253] | |
| Solanum nigrum methanolic leaves extract | In vivo Oral glucose tolerance test in wistar albino rats | 4–25 nm | Significant decrease in the blood glucose level by 10 mg/kg 2 h after (comparable with glibenclamide) and improvement in the body weight after 21 days | [250] | |
| Zingiber officinales ethanolic extract | In vivo antidiabetic activity in streptozocin-induced diabetic rats | 123.8 nm | Decreasing of blood glucose level to normal from 249 ± 0.67 mg/dL to 86 ± 0.91 mg/dL on 7th day after the administration by 200 mg/kg of body weight similar to metformin 82 ± 1.9 mg/dL | [251] | |
| Zinc oxide nanoparticles (ZnONPs) | Azadirachta indica, Hibiscus rosa-sinensis, Murraya koenigii, Moringa oleifera, and Tamarindus indica aqueous leaves extract | In vitro α-amylase and α-glucosidase inhibitor activity and antioxidant activity | 27–54 nm | Exhibition of appreciable α-amylase and α-glucosidase inhibitory activity compared with chemically synthesized ZnO nanoparticles and enhanced antioxidant activity contrarily to chemically synthesized ZnO nanoparticles | [254] |
| Hibiscus subdariffa leaf aqueous extract | In vivo antidiabetic activity on streptozocin-induced diabetic mice | 12–46 nm | Suitable antidiabetic effect of ZnONPS (at a dose of 8 mg/kg per day for a period of 28 days): reduction in glycemia in mice treated up to 59.58% than that of untreated | [255] | |
| Vaccinium arctostaphylus L fruit ethanolic extract 96% | In vivo antidiabetic effect on alloxan-induced diabetic rats | 15 nm | High efficacy of biosynthesized ZnONPs (8 mg/dL). Most effective reduction in fasting blood glucose to 50.4 ± 3.55 than the extract (150 mg/dL) 79.4 ± 2.85 more than chemically synthesized (8 mg/dL) 83.2 ± 9.54 more than insulin (10 UI/kg) 113.8 ± 12.96 in treating the alloxan-diabetic rats daily for 16 days compared to diabetic non-treated rats 199.8 ± 10.94 mg/dL | [244] | |
| Silybum marianum L aqueous seeds extract | In vivo antidiabetic activity on alloxan-induced diabetic wistar rats | 19.9 nm | Decrease in fasting blood sugar from 207 mg/dL to 96 mg/dl by 10 (mg/dL) of green-synthesized ZnO after 16 days of treatment more than the extract (150 mg/dL) and then the insulin 10 UI/kg and insulin level increased significantly to 1,7 µg/dL compared to rat receiving chemical ZnONPS and the extract (about 0,9 µg/dL) | [237] | |
| Costus igneus aqueous leaves extract | In vitro α-amylase and α-glucosidase inhibition assays | 26.55 nm | Suitable antidiabetic and antioxidant activity α-amylase and α-glucosidase inhibition activity of synthesized zinc oxide nanoparticles was 74% and 82%, respectively, and the 2,2-diphenyl-1-picrylhydrazyl hydrate reducing power at 100 μg/mL was 75% | [256] | |
| Eryngium billardieri aqueous Leaf Extract | In vivo alloxan-induced diabetic rats antidiabetic activities | 34 nm | The green ZnONPs 7 mg/kg daily for 16 days showed an excellent efficiency in overcoming diabetic rise of high-density lipoprotein and greatly reduced fasting blood sugar levels to 103.2 mg/dL compared to the extract alone 116.9 mg/dL and the chemically synthesized zinc oxide nanoparticles 114.1 mg/dL, cholesterol reduction, and increased insulin levels 1.29 µg/dL than the extract 0.48 µg/dL and the chemically synthesized zinc oxide nanoparticles 0.48 µg/dL | [245] | |
| Selenium nanoparticles (SeNPs) | Hibiscus sabdariffa aqueous leaf extract | Evaluation of factors correlated to oxidative damage in in vivo streptozocin-induced diabetic Wistar rats | ND | 1 mg of synthesized SeNPs/10 kg body weight, given once per day for a period of 28 days elevated antioxidant enzyme activities and the glutathione value (to more than 0.35 mmol/mg protein) in testicular tissues than insulin treatment (less than 0.25 mmol/mg protein) | [257] |
| Mulberry leaf and Pueraria lobata ethanolic extracts | In vivo hypoglycemic effect in diabetic rats | 120 nm | Significant hypoglycemic effects with decrease in blood glucose up to 32.88% compared to plant extracts and nanoparticles alone at a dose of 125 mg/kg. Synergistic effect between plant extracts and SeNPs | [258] | |
| Catathelasma ventricosum polysaccharides solution | Antidiabetic activity in streptozocin-induced diabetic male outbred stock from Institute of Cancer Research (ICR) mice | 50 nm | Higher antidiabetic activity than chemical selenium NPs, selenium salt, and the extract alone 2 mg/kg daily for 30 consecutive days. Decrease in glycemia from 24 to 8.3 mmol/L, 24.4 to 17.7 mmol/L, and 24.4 to 12.6 mmol/L, respectively Synergistic effect between plant extracts and SeNPs. Improvement in body weight, blood sugar, antioxidant enzymes activities, and lipid levels | [259] | |
| Gold nanoparticles (AuNPs) | Leucosidea sericea procyanidins fractions | In vitro α-amylase and α-glucosidase inhibitory activity | 6–24 nm | Stronger α-amylase inhibitory activity (IC50 value at 1.88 µg/mL) and α-glucosidase activity (IC50 value at 4.5 µg/mL) than the fractions alone (IC50 value at 3.5 ± 0.7 µg/mL and 8.1 ± 0.6 µg/mL, respectively), more efficient than acarbose (610 ± 2.6 µg/mL and 10.2 ± 0.6 µg/mL, respectively) | [260] |
| Eclipta alba methaolic extract | In vitro evaluation of prevention of β-cell damage (RIN-5F pancreatic β-cells) | 26 nm | Anti-apoptotic potential of β-cell decreased cell damage. Decreased level of Bcl-2 protein and increased level of Bax indicates the cell survival induced by NF-κB pathway | [261] | |
| Fritillaria cirrhosa aqueous extract | In vivo antidiabetic effect in streptozotocin diabetic rats | 40–45 nm | 20 mg/kg per day for 28 days decreased blood glucose to just under 150 mg/dL in comparison to controlling glycemia by about 300 mg/dL and increased insulin level. A decrease in glycosylated hemoglobin, modulation of antioxidants level, decrease in lipid peroxidation, and regeneration of islets cells were seen | [262] | |
| Cassia auriculata propanoic acid 2-(3-acetoxy-4,4,14-trimethylandrost-8-en-17-yl) (PAT) | In vivo antidiabetic activity in alloxan-induced diabetic rats | 12–41 nm | Green-synthesized gold nanoparticles (0.5 mg/kg daily for 28 days) significantly decreased the plasma glucose level to lower than 150 mg/dL in comparison to diabetic control, which was more than 300 mg/dL. Cholesterol levels, as well as triglyceride levels, decreased significantly, and an elevation of insulinemia was shown with synthesized nanoparticles | [263] | |
| Sargassum swartzii extract | In vivo antidiabetic effect using male wistar Albino rats | 37 nm | Green-synthesized gold nanoparticles (0.5 mg/kg per day orally for 28 days) significantly decreased fasting blood glucose levels to 98 ± 7.2 mg/dl compared to diabetic control 218 ± 9.6 mg/dL. Glycosylated hemoglobin levels decreased to 0.34±0.041 mg/g Hb compared to diabetic control 0.84 ± 0.05. Insulin levels increased in the diabetic rats receiving green-synthesized gold nanoparticles | [264] | |
| Cassia fistula stem bark aqueous extract | In vivo antidiabetic streptozotocin-induced diabetic rats | 55.2–98.4 nm | Green-synthesized gold nanoparticles (60 mg/kg bw oral gavage) decreased serum blood glucose concentrations to 168.47 ± 16.18 mg/dL by synthesized nanoparticles in comparison to rats treated with the extract (60 mg/kg bw) 211.05 ± 5.40 mg/dL and HbA1c levels to 10.40% ± 0.23% and 11.45% ± 0.28%, respectively. Improvement in body conditions and the activity of transaminase enzymes, Upregulation of lipid profile, reversion of kidney failure | [265] | |
| Moringa oliefera leaves aqueous extract | In vitro α-amylase inhibition activity | 15.2 nm | Inhibition of the enzyme in a concentration-dependent mode IC50 value of 130 µg/mL | [266] | |
| Copper nanoparticles (CuNPs) | Dioscorea bulbifera extract | In vitro α-glucosidase inhibition activity | 12–16 nm | 99.1 ± 0.2% and 90.7 ± 0.3% suppression of α-glucosidase and murine intestinal glucosidase activity, respectively | [239] |
| Millettia pinnata aqueous extract of the flower | In vitro α antidiabetic activity | 23 ± 1.10 nm | Inhibition of α-amylase in a dose-dependent manner Constrained glycosylation at 77% compared with the extract solution (54%) High antioxidant property offered by extract functional groups | [267] | |
| Gnidia glauca leaves aqueous extracts | In vitro α glucosidase inhibition assay | 70–93 nm | Inhibition of 88.60 ± 0.78% at 100 μg/mL | [268] | |
| Bacopa monnieri leaf extract | In vivo antidiabetic effect in streptozocin-induced diabetic mice | 34.4 nm | Single dose of synthesized CuNPs at 14 mg/kg by oral route and 7 mg/kg orally accompanied by subcutaneous 0.2 U of insulin per 50 g decreased blood glucose levels by almost 33.7 and 32.2%, respectively | [269] | |
| Iron nanoparticles (FeNPs) | Polyherbal Formulation Containing Tinospora cordiofolia, Curcuma longa Trigonella foenum gracum, Emblica officinale, and Salacia oblonga methanolic extract | In vitro α-amylase inhibitory assay | 40–60 nm | Equivalent α amylase enzyme inhibitory activity (70.48%) to the standard ascorbic acid (73.87%) at the maximum concentration of 250 µg/mL | [270] |
| Sesamum indicum aqueous seeds extract | In vitro α-amylase inhibitory assay | 99 nm | α-amylase inhibition activity was shown in a dose-dependent manner (10–100 µg/mL). The highest inhibitory activity, was 64.39 ± 0.52% with IC50 value = 21.26 μg/mL | [271] | |
| Platinum nanoparticles (PtNPs) | Polygonum salicifolium leaves aqueous extract | In vitro α-amylase and α-glucosidase inhibition assay | 1–3 nm | Remarkable suppression of α-glucosidase activity with IC50 = 53 μg/mL in comparison to that of acarbose and curcumin and slight α-amylase inhibitory effect | [272] |
| Whitania somnifera leaves | In vivo antidiabetic activity in streptozotocin-induced diabetic rats | 12 nm | 1 mg/kg PtNPs decreased plasma glucose levels to the normal level (117.34 ± 4.18 mg/dL) than intraperitoneal injection of 10 mL/kg plant extract (153.34 ± 8.16 mg/dL) compared to the control group (453.34 ± 8.16 mg/dL) after 28 days treatment | [273] | |
| Platinum-titanium oxide nanoparticles (Pt-TiO2NPs) | Costus speciosus 99% ethanolic leaf extract | α amylase inhibition test | ND | Suitable α-amylase inhibition effect. Costus speciosus-Pt-TiO2NPs at 1% and 3% showed α-amylase inhibition of 71.1 ± 1.0% and 77.4 ± 1.7%, respectively, at a dose of 100 µg/mL | [274] |
| Titanium dioxide nanoparticles (TiO2NPs) | Azadirachta indica leaves aqueous extract | α-amylase inhibition assay | ND | Up to 97.2% inhibition was produced by TiO2NPs at 50 µg/mL, which was similar to the effect exhibited by acarbose | [275] |
| Stevia rebaudiana (Bertoni) Bertoni leaves alcohol-aqueous (80:20) extract | Hypoglycemic effect in alloxan-induced diabetic rats | 100 nm | Induction of strong and sustained reduction in the glucose level up to 30 days after green-synthesized TiO2NPs administration at a dose of 1 g/kg intraperitoneally (solution 20 and 30 μM). The glycemia was lowered by 20% and 56%, and glycated hemoglobin by 25% and 41% compared to the reduction by the chemically synthesized TiO2NPs | [276] | |
| Palladium oxide nanoparticles (PdONPs) | Zanthoxylum armatum fruit aqueous extract | α-glucosidase inhibition assay | 10.5 nm | Excellent enzyme inhibition with IC50 = 0.0218 ± 0.01 μg/mL | [277] |
| Nickel oxide nanoparticles (NiONPs) | Areca catechu leaf extract | α-amylase inhibition assay | 5.46 nm | NiONPs showed an inhibition of the enzyme with IC 50 value = 268.13 µg/mL | [278] |
| Cerium oxide (CeO), zinc oxide, and silver nanoparticles | Momordica charantia fruits extract | In vivo antidiabetic effect in streptozotocin-induced diabetic rats | 24 nm | 200 mg/kg per day for 15 days of synthesized nanoparticles: cerium oxide, zinc oxide, and silver decreased blood glucose to 179.78 ± 5.12 mg/dL, 132.07 ± 6.21 mg/dL and 103.07 ± 6.21 mg/dL, respectively, and extract to 214.98 ± 3.43 mg/dL in comparison to the control 268.89 ± 3.76 mg/dL | [243] |
| Cerium oxide nanoparticles (CeONPs) | fruit extract of Morus nigra | In vitro glucose uptake of L6 cell lines | 8.5 nm | 100 μg/mL CeONPs enhanced glucose uptake to more than 65% higher than untreated cells | [279] |
4.2.2. Green-Synthesized Zinc Oxide Nanoparticles
4.2.3. Green-Synthesized Selenium Nanoparticles
4.2.4. Green-Synthesized Gold Nanoparticles
4.2.5. Green-Synthesized Iron Nanoparticles
4.2.6. Green-Synthesized Copper Nanoparticles
4.2.7. Green-Synthesized Cerium Nanoparticles
4.2.8. Green-Synthesized Platinum Nanoparticles
4.2.9. Green-Synthesized Titanium Nanoparticles
4.2.10. Other Green-Synthesized Inorganic Nanoparticles
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas 2021, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; ISBN 9782930229980. [Google Scholar]
- Of, D.; Mellitus, D. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Potential of Insulin Nanoparticle Formulations for Oral Delivery and Diabetes Treatment. J. Control. Release 2017, 264, 247–275. [Google Scholar] [CrossRef]
- Singh, A.; Teixeira, M. Modern Medicine: Towards Prevention, Cure, Well-Being and Longevity. Rev. Latinoam. Psicopatol. Fundam. 2010, 13, 265–282. [Google Scholar] [CrossRef] [Green Version]
- Souto, E.B.; Souto, S.B.; Campos, J.R.; Severino, P.; Pashirova, T.N.; Zakharova, L.Y.; Silva, A.M.; Durazzo, A.; Lucarini, M.; Izzo, A.A.; et al. Nanoparticle Delivery Systems in the Treatment of Diabetes Complications. Molecules 2019, 24, 4209. [Google Scholar] [CrossRef] [Green Version]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The Role of Medicinal Plants in the Treatment of Diabetes: A Systematic Review. Electron. Phys. 2016, 8, 1832–1842. [Google Scholar] [CrossRef] [Green Version]
- Bindu, J.; Narendhirakannan, R.T. Role of Medicinal Plants in the Management of Diabetes Mellitus: A Review. 3 Biotech 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Bährle-Rapp, M. Semen Hippocastani. In Springer Lexikon Kosmetik und Körperpflege; Spinger: Berlin/Heidelberg, Germany, 2007; p. 499. [Google Scholar]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; ul Ain, N.; Ao, Q. Role of Capping Agents in the Application of Nanoparticles in Biomedicine and Environmental Remediation: Recent Trends and Future Prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Kovács, D.; Igaz, N.; Vágvölgyi, C.; Boros, I.M.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Biological Activity of Green-Synthesized Silver Nanoparticles Depends on the Applied Natural Extracts: A Comprehensive Study. Int. J. Nanomed. 2017, 2017, 871–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, X.; Chen, Z.; Pang, L.; Wang, L.; Jiang, H.; Chen, Y.; Zhang, Z.; Fu, C.; Ren, B.; Zhang, J. Oral Nano Drug Delivery Systems for the Treatment of Type 2 Diabetes Mellitus: An Available Administration Strategy for Antidiabetic Phytocompounds. Int. J. Nanomed. 2020, 15, 10215–10240. [Google Scholar] [CrossRef] [PubMed]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes Mellitus and Oxidative Stress—A Concise Review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; Karuranga, S., da Fernandes, J.R., Huang, Y., Eds.; International Diabetes Federation: Brussels, Belgium, 2017; ISBN 9782930229874. [Google Scholar]
- American Diabetes Association. Standards of Medical Care in Diabetes—2016 Abridged for Primary Care Providers. Clin. Diabetes 2016, 34, 3–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazi, A.A.; Blonde, L. Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 2001; Volume 21, ISBN 9789241515702. [Google Scholar]
- World Health Organization 2016. Global Report on Diabetes. Available online: https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;Sequence=1 (accessed on 6 October 2022).
- Vieira, R.; Souto, S.B.; Sánchez-López, E.; Machado, A.L.; Severino, P.; Jose, S.; Santini, A.; Silva, A.M.; Fortuna, A.; García, M.L.; et al. Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome—Strategies for in Vivo Administration: Part-II. J. Clin. Med. 2019, 8, 1332. [Google Scholar] [CrossRef] [Green Version]
- Berenson, D.F.; Weiss, A.R.; Wan, Z.; Weiss, M.A. Insulin Analogs for the Treatment of Diabetes Mellitus: Therapeutic Applications of Protein Engineering. Ann. N. Y. Acad. Sci. 2011, 1243, E40–E54. [Google Scholar] [CrossRef] [Green Version]
- Chaudhury, A.; Duvoor, C.; Dendi, V.S.R.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Edelman, S.; Maier, H.; Wilhelm, K. Pramlintide in the Treatment of Diabetes Mellitus. BioDrugs 2008, 22, 375–386. [Google Scholar] [CrossRef]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Medical Management of Hyperglycemia in Type 2 Diabetes: A Consensus Algorithm for the Initiation and Adjustment of Therapy. Diabetes Care 2009, 32, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Hughes, S.; Neumiller, J.J. Oral Semaglutide. Clin. Diabetes 2020, 38, 109–111. [Google Scholar] [CrossRef]
- Pirson, N.; Maiter, D.; Alexopoulou, O. EndocrinologiE Et Nutrition. Louvain Med. 2016, 135, 661–668. [Google Scholar]
- Salehi, B.; Ata, A.; Kumar, N.V.A.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [Green Version]
- De Sousa, E.; Zanatta, L.; Seifriz, I.; Creczynski-Pasa, T.B.; Pizzolatti, M.G.; Szpoganicz, B.; Silva, F.R.M.B. Hypoglycemic Effect and Antioxidant Potential of Kaempferol-3,7-O-(α)-Dirhamnoside from Bauhinia Forficata Leaves. J. Nat. Prod. 2004, 67, 829–832. [Google Scholar] [CrossRef]
- Mooney, M.H.; Fogarty, S.; Stevenson, C.; Gallagher, A.M.; Palit, P.; Hawley, S.A.; Hardie, D.G.; Coxon, G.D.; Waigh, R.D.; Tate, R.J.; et al. Mechanisms Underlying the Metabolic Actions of Galegine That Contribute to Weight Loss in Mice. Br. J. Pharmacol. 2008, 153, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, A.C.M.; Frode, T.S. Isolated Compounds from Natural Products with Potential Antidiabetic Activity—A Systematic Review; Bentham Scienece Publisher: Sharjah, United Arab Emirates, 2017; Volume 14, ISBN 6661705051. [Google Scholar]
- Blaschek, W. Natural Products as Lead Compounds for Sodium Glucose Cotransporter (SGLT) Inhibitors. Planta Med. 2017, 83, 985–993. [Google Scholar] [CrossRef]
- Moradi-Marjaneh, R.; Paseban, M.; Sahebkar, A. Natural Products with SGLT2 Inhibitory Activity: Possibilities of Application for the Treatment of Diabetes. Phyther. Res. 2019, 33, 2518–2530. [Google Scholar] [CrossRef]
- Schiff, P.L. Opium and Its Alkaloids. Am. J. Pharm. Educ. 2002, 66, 186–194. [Google Scholar]
- Feldo, M.; Woźniak, M.; Wójciak-Kosior, M.; Sowa, I.; Kot-Waśik, A.; Aszyk, J.; Bogucki, J.; Zubilewicz, T.; Bogucka-Kocka, A. Influence of Diosmin Treatment on the Level of Oxidative Stress Markers in Patients with Chronic Venous Insufficiency. Oxid. Med. Cell. Longev. 2018, 2018, 2561705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine; World Health Organization: Geneva, Switzerland, 2019; Volume 2019, ISBN 9789241515436. [Google Scholar]
- Mokgobi, M.G. Understanding Traditional African Healing. Afr. J. Phys. Health Educ. Recreat. Dance 2014, 20, 24–34. [Google Scholar]
- Ozioma, E.-O.J.; Chinwe, O.A.N. Herbal Medicine. In Herbal Medicine; IntechOpen: Vienna, Austria, 2019. [Google Scholar] [CrossRef] [Green Version]
- Chawla, R.; Thakur, P.; Chowdhry, A.; Jaiswal, S.; Sharma, A.; Goel, R.; Sharma, J.; Priyadarshi, S.S.; Kumar, V.; Sharma, R.K.; et al. Evidence Based Herbal Drug Standardization Approach in Coping with Challenges of Holistic Management of Diabetes: A Dreadful Lifestyle Disorder of 21st Century. J. Diabetes Metab. Disord. 2013, 12, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musa, D.A.; Dim-Gbereva, L.; Ogbiko, C.; Nwodo, O.F.C. Phytochemical and in Vitro Anti-Typhoid Properties of Leaf, Stem and Root Extracts of Ficus Capensis (Moraceae). J. Pharm. Bioresour. 2019, 16, 165. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. A Review on Ethnopharmacological Applications, Pharmacological Activities, and Bioactive Compounds of Mangifera Indica (Mango). Evid. Based Complement. Altern. Med. 2017, 2017, 6949835. [Google Scholar] [CrossRef] [Green Version]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, H.; Singh, S.C. Green Synthesis of Nanoparticles and Its Potential Application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019—Google Livres. Available online: https://books.google.be/books?hl=fr&lr=&id=WHOyDwAAQBAJ&oi=fnd&pg=PP1&ots=h2flx08Vnz&sig=Y4Mwfz4wGsmKZ_dnb8jhUdmJ4JA&redir_esc=y#v=onepage&q&f=false (accessed on 22 September 2021).
- Rambhade, S.; Chakraborty, A.; Patil, U.; Rambhade, A. Journal of Chemical and Pharmaceutical Research Preparations. J. Chem. Pharm. Res. 2010, 2, 7–25. [Google Scholar]
- Governa, P.; Baini, G.; Borgonetti, V.; Cettolin, G.; Giachetti, D.; Magnano, A.R.; Miraldi, E.; Biagi, M. Phytotherapy in the Management of Diabetes: A Review. Molecules 2018, 23, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.Y.; Aziz, I.; Bihari, B.; Kumar, H.; Roy, M.; Verma, V.K. A review- phytomedicines used in treatment of diabetes. IJP 2014, 1, 343–365. [Google Scholar]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as α-Amylase and α-Glucosidase Inhibitors and Their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Dewanjee, S.; Chakraborty, P.; Mukherjee, B.; de Feo, V. Plant-Based Antidiabetic Nanoformulations: The Emerging Paradigm for Effective Therapy. Int. J. Mol. Sci. 2020, 21, 2217. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhu, J.; Li, Z.; Zhu, W.; Shi, J.; Jia, Q.; Li, Y. Recent Progress in Natural Products as DPP-4 Inhibitors. Future Med. Chem. 2015, 7, 1079–1089. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, J.; Ji, C. Quercetin: A Potential Drug to Reverse Multidrug Resistance. Life Sci. 2010, 87, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Birhman, K.; Raheja, I.; Sharma, S.K.; Kar, H.K. Quercetin: A Wonder Bioflavonoid with Therapeutic Potential in Disease Management. Asian Pac. J. Trop. Dis. 2016, 6, 248–252. [Google Scholar] [CrossRef]
- Yao, Z.; Gu, Y.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Xia, Y.; Bao, X.; Shi, H.; Sun, S.; et al. Estimated Daily Quercetin Intake and Association with the Prevalence of Type 2 Diabetes Mellitus in Chinese Adults. Eur. J. Nutr. 2019, 58, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.J.; Li, Y.; Cao, Q.H.; Wu, H.X.; Tang, X.Y.; Gao, X.H.; Yu, J.Q.; Chen, Z.; Yang, Y. In Vitro and in Vivo Evidence That Quercetin Protects against Diabetes and Its Complications: A Systematic Review of the Literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Ahmed, Z.A.; Mahwi, T.O.; Aziz, T.A. Effect of Quercetin on Postprandial Glucose Excursion after Mono- and Disaccharides Challenge in Normal and Diabetic Rats. J. Diabetes Mellit. 2012, 02, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Ostadmohammadi, V.; Milajerdi, A.; Ayati, E.; Kolahdooz, F.; Asemi, Z. Effects of Quercetin Supplementation on Glycemic Control among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phyther. Res. 2019, 33, 1330–1340. [Google Scholar] [CrossRef]
- Han, M.K.; Barreto, T.A.; Martinez, F.J.; Comstock, A.T.; Sajjan, U.S. Randomised Clinical Trial to Determine the Safety of Quercetin Supplementation in Patients with Chronic Obstructive Pulmonary Disease. BMJ Open Respir. Res. 2020, 7, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, T. Safety of Quercetin for Clinical Application. Int. J. Mol. Med. 2005, 16, 275–278. [Google Scholar] [CrossRef]
- Murota, K.; Terao, J. Antioxidative Flavonoid Quercetin: Implication of Its Intestinal Absorption and Metabolism. Arch. Biochem. Biophys. 2003, 417, 12–17. [Google Scholar] [CrossRef]
- Chen, X.; Yin, O.Q.P.; Zuo, Z.; Chow, M.S.S. Pharmacokinetics and Modeling of Quercetin and Metabolites. Pharm. Res. 2005, 22, 892–901. [Google Scholar] [CrossRef]
- Suresh, K.; Nangia, A. Curcumin: Pharmaceutical Solids as a Platform to Improve Solubility and Bioavailability. CrystEngComm 2018, 20, 3277–3296. [Google Scholar] [CrossRef]
- Abu-Taweel, G.M.; Attia, M.F.; Hussein, J.; Mahmoud, E.; Galal, H.M.; Ibrahim, E.; Allam, A.A.; El-naggar, M.E. Biomedicine & Pharmacotherapy Curcumin Nanoparticles Have Potential Antioxidant Effect and Restore Tetrahydrobiopterin Levels in Experimental Diabetes. Biomed. Pharmacother. 2020, 131, 110688. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.T.; Pescinini-e-Salzedas, L.M.; Camargo, M.E.C.; Barbalho, S.M.; dos Haber, J.F.S.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; Cincotto dos Santos Bueno, P. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front. Endocrinol. 2021, 12, 669448. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Reiner, Ž.; Majeed, M.; Sahbekar, A. Curcuminoids Modify Lipid Profile in Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Complement. Ther. Med. 2017, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Behnam, B.; Mohammadinejad, R.; Guest, P.C.; Simental-Mendía, L.E.; Sahebkar, A. Antidiabetic Properties of Curcumin: Insights on New Mechanisms. Adv. Exp. Med. Biol. 2021, 1291, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Na, L.X.; Li, Y.; Pan, H.Z.; Zhou, X.L.; Sun, D.J.; Meng, M.; Li, X.X.; Sun, C.H. Curcuminoids Exert Glucose-Lowering Effect in Type 2 Diabetes by Decreasing Serum Free Fatty Acids: A Double-Blind, Placebo-Controlled Trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [Green Version]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An Anti-Inflammatory Molecule from a Curry Spice on the Path to Cancer Treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef]
- Yang, K.Y.; Lin, L.C.; Tseng, T.Y.; Wang, S.C.; Tsai, T.H. Oral Bioavailability of Curcumin in Rat and the Herbal Analysis from Curcuma Longa by LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 853, 183–189. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Sun, Y.; Zhang, G.; Bai, J.; Guo, J.; Su, X.; Du, H.; Cao, X.; Yang, J.; et al. Naringenin Improves Insulin Sensitivity in Gestational Diabetes Mellitus Mice through AMPK. Nutr. Diabetes 2019, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Wojnar, W.; Zych, M.; Kaczmarczyk-Sedlak, I. Antioxidative Effect of Flavonoid Naringenin in the Lenses of Type 1 Diabetic Rats. Biomed. Pharmacother. 2018, 108, 974–984. [Google Scholar] [CrossRef]
- Qi, Z.; Xu, Y.; Liang, Z.; Li, S.; Wang, J.; Wei, Y.; Dong, B. Naringin Ameliorates Cognitive Deficits via Oxidative Stress, Proinflammatory Factors and the PPARγ Signaling Pathway in a Type 2 Diabetic Rat Model. Mol. Med. Rep. 2015, 12, 7093–7101. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Bharti, S.; Ojha, S.; Bhatia, J.; Kumar, N.; Ray, R.; Kumari, S.; Arya, D.S. Up-Regulation of PPARγ, Heat Shock Protein-27 and-72 by Naringin Attenuates Insulin Resistance, β-Cell Dysfunction, Hepatic Steatosis and Kidney Damage in a Rat Model of Type 2 Diabetes. Br. J. Nutr. 2011, 106, 1713–1723. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the Citrus Flavanone Aglycones Hesperetin and Naringenin after Single Oral Administration in Human Subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Wu, H.; Wang, Y.; Peng, W.; Su, W. Toxicological Evaluation of Naringin: Acute, Subchronic, and Chronic Toxicity in Beagle Dogs. Regul. Toxicol. Pharmacol. 2020, 111, 104580. [Google Scholar] [CrossRef]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2021, 22, 91–98. [Google Scholar] [CrossRef]
- Nanjan, M.J.; Betz, J. Resveratrol for the Management of Diabetes and Its Downstream Pathologies. Eur. Endocrinol. 2014, 10, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szkudelski, T.; Szkudelska, K. Resveratrol and Diabetes: From Animal to Human Studies. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thadhani, V.M. Resveratrol in Management of Diabetes and Obesity: Clinical Applications, Bioavailability, and Nanotherapy. In Resveratrol—Adding Life to Years, Not Adding Years to Life; IntechOpen: Vienna, Austria, 2019. [Google Scholar] [CrossRef] [Green Version]
- Movahed, A.; Nabipour, I.; Louis, X.L.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M.; Rekabpour, S.J.; Netticadan, T. Antihyperglycemic Effects of Short Term Resveratrol Supplementation in Type 2 Diabetic Patients. Evid. Based Complement. Altern. Med. 2013, 2013, 851267. [Google Scholar] [CrossRef] [Green Version]
- Tani, H.; Hikami, S.; Iizuna, S.; Yoshimatsu, M.; Asama, T.; Ota, H.; Kimura, Y.; Tatefuji, T.; Hashimoto, K.; Higaki, K. Pharmacokinetics and Safety of Resveratrol Derivatives in Humans after Oral Administration of Melinjo (Gnetum Gnemon L.) Seed Extract Powder. J. Agric. Food Chem. 2014, 62, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic Biol Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Zhao, Z.; Moghadasian, M.H. Chemistry, Natural Sources, Dietary Intake and Pharmacokinetic Properties of Ferulic Acid: A Review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Matuo, T.; Tsuno, T.; Hosoda, A.; Nomura, E.; Taniguchi, H.; Sasaki, H.; Morishita, H. Antioxidant Activity and Hypoglycemic Effect of Ferulic Acid in STZ-Induced Diabetic Mice and KK-Ay Mice. BioFactors 2004, 21, 315–319. [Google Scholar] [CrossRef]
- Nomura, E.; Kashiwada, A.; Hosoda, A.; Nakamura, K.; Morishita, H.; Tsuno, T.; Taniguchi, H. Synthesis of Amide Compounds of Ferulic Acid, and Their Stimulatory Effects on Insulin Secretion in Vitro. Bioorg. Med. Chem. 2003, 11, 3807–3813. [Google Scholar] [CrossRef]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Insulin-Signalling Molecules in the Liver of High-Fat Diet and Fructose-Induced Type-2 Diabetic Adult Male Rat. Appl. Physiol. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic Acid: Pharmacological and Toxicological Aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef] [Green Version]
- Ou, S.; Kwok, K.C. Ferulic Acid: Pharmaceutical Functions, Preparation and Applications in Foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Zhao, Z.; Egashira, Y.; Sanada, H. Ferulic Acid Is Quickly Absorbed from Rat Stomach as the Free Form and Then Conjugated Mainly in Liver. J. Nutr. 2004, 134, 3083–3088. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, C.; Zhang, Y.; Mi, S.; Wang, N. Pharmacokinetics of Ferulic Acid and Potential Interactions with Honghua and Clopidogrel in Rats. J. Ethnopharmacol. 2011, 137, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A Comprehensive Review on Its Biological Potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef]
- Maronpot, R.R.; Koyanagi, M.; Davis, J.; Recio, L.; Marbury, D.; Boyle, M.; Hayashi, S.M. Safety Assessment and Single-Dose Toxicokinetics of the Flavouring Agent Myricitrin in Sprague–Dawley Rats. Food Addit. Contam. A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-Glucosidase and α-Amylase by Flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, F.; Ozmen, A.; Akkaya, B.; Aliciguzel, Y.; Aslan, M. Beneficial Effect of Myricetin on Renal Functions in Streptozotocin-Induced Diabetes. Clin. Exp. Med. 2012, 12, 265–272. [Google Scholar] [CrossRef]
- Ong, K.C.; Khoo, H.E. Effects of Myricetin on Glycemia and Glycogen Metabolism in Diabetic Rats. Life Sci. 2000, 67, 1695–1705. [Google Scholar] [CrossRef]
- Park, K.S.; Chong, Y.; Kim, M.K. Myricetin: Biological Activity Related to Human Health. Appl. Biol. Chem. 2016, 59, 259–269. [Google Scholar] [CrossRef]
- Dang, Y.; Lin, G.; Xie, Y.; Duan, J.; Ma, P.; Li, G.; Ji, G. Quantitative Determination of Myricetin in Rat Plasma by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry and Its Absolute Bioavailability. Drug Res. 2013, 64, 516–522. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Yu, J.; Liu, C.; Li, L.; Zhang, Y. Isolation of Liquiritigenin-4′-Apiosyl-Glucoside and Liquiritin from the Root of Glycyrrhiza Uralensis by High-Performance Centrifugal Partition Chromatography. J. Chromatogr. Sci. 2014, 52, 310–314. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Chen, J.; Peng, F.; Sun, C.; Lei, Y.; Chen, G.; Li, G.; Yin, Y.; Lin, Z.; Wu, L.; et al. Pharmacological Activities and Pharmacokinetics of Liquiritin: A Review. J. Ethnopharmacol. 2022, 293, 115257. [Google Scholar] [CrossRef]
- Modak, M.; Dixit, P.; Londhe, J.; Ghaskadbi, S.; Devasagayam, T.P.A. Indian Herbs and Herbal Drugs Used for the Treatment of Diabetes. J. Clin. Biochem. Nutr. 2007, 40, 163–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Jiang, Y.; Zhang, Z.; Hou, J.; Tian, S.; Liu, Y. The Anti-Diabetic Activity of Licorice, a Widely Used Chinese Herb. J. Ethnopharmacol. 2020, 263, 113216. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zhang, Y.; Xu, J.; Sun, L.; Li, S. The Protective Role of Liquiritin in High Fructose-Induced Myocardial Fi Brosis via Inhibiting NF- k B and MAPK Signaling Pathway. Biomed. Pharmacother. 2016, 84, 1337–1349. [Google Scholar] [CrossRef]
- Han, Y.J.; Kang, B.; Yang, E.; Choi, M. Simultaneous Determination and Pharmacokinetic Characterization of Glycyrrhizin, Isoliquiritigenin, Liquiritigenin, and Liquiritin in Rat Plasma Following Oral Administration of Glycyrrhizae. Molecules 2019, 24, 1816. [Google Scholar] [CrossRef]
- Zhu, J.; Deng, Y.-Q.; Wang, X.; Li, X.-F.; Zhang, N.-N.; Liu, Z.; Zhang, B.; Qin, C.-F.; Xie, Z. An Artificial Intelligence System Reveals Liquiritin Inhibits SARS-CoV-2 by Mimicking Type I Interferon. bioRxiv 2020. [Google Scholar] [CrossRef]
- Chae, B.S. Protective Effect of Baicalin on the TNF-??-Mediated Development of Insulin Resistance in Differentiated 3T3-L1 Cells. Nat. Prod. Sci. 2013, 19, 316–323. [Google Scholar]
- Ku, S.K.; Bae, J.S. Baicalin, Baicalein and Wogonin Inhibits High Glucose-Induced Vascular Inflammation in Vitro and in Vivo. BMB Rep. 2015, 48, 519–524. [Google Scholar] [CrossRef]
- Kuo, Y.T.; Lin, C.C.; Kuo, H.T.; Hung, J.H.; Liu, C.H.; Jassey, A.; Yen, M.H.; Wu, S.J.; Lin, L.T. Identification of Baicalin from Bofutsushosan and Daisaikoto as a Potent Inducer of Glucose Uptake and Modulator of Insulin Signaling-Associated Pathways. J. Food Drug Anal. 2019, 27, 240–248. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Liu, X.; Sun, J. Inhibitory Mechanism of Baicalein against α-Glucosidase. Nat. Prod. Commun. 2019, 14, 1–5. [Google Scholar] [CrossRef]
- Wang, G.; Liang, J.; Gao, L.R.; Si, Z.P.; Zhang, X.T.; Liang, G.; Yan, Y.; Li, K.; Cheng, X.; Bao, Y.; et al. Baicalin Administration Attenuates Hyperglycemia-Induced Malformation of Cardiovascular System Article. Cell Death Dis. 2018, 9, 234. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Gao, H.; Lou, K.; Luo, H.; Hao, S.; Yuan, J.; Liu, Z.; Dong, R. Safety, Tolerability, and Pharmacokinetics of Oral Baicalein Tablets in Healthy Chinese Subjects: A Single-Center, Randomized, Double-Blind, Placebo-Controlled Multiple-Ascending-Dose Study. Clin. Transl. Sci. 2021, 14, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Sune, E.; Song, J.; Wang, J.; Jia, X.B.; Zhang, Z.H. Characterization and Bioavailability Study of Baicalin-Mesoporous Carbon Nanopowder Solid Dispersion. Pharmacogn. Mag. 2016, 12, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Liu, Y.; Zhang, C. Pharmacokinetics and Bioavailability Enhancement of Baicalin: A Review. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Chueh, W.H.; Lin, J.Y. Berberine, an Isoquinoline Alkaloid in Herbal Plants, Protects Pancreatic Islets and Serum Lipids in Nonobese Diabetic Mice. J. Agric. Food Chem. 2011, 59, 8021–8027. [Google Scholar] [CrossRef]
- Yin, J.; Ye, J.; Jia, W. Effects and Mechanisms of Berberine in Diabetes Treatment. Acta Pharm. Sin. B 2012, 2, 327–334. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of Berberine in Patients with Type 2 Diabetes Mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.J.; Zhang, H.; Song, D.Q.; Xue, R.; Zhao, W.; Wei, J.; Wang, Y.M.; Shan, N.; Zhou, Z.X.; Yang, P.; et al. Berberine Reduces Insulin Resistance through Protein Kinase C-Dependent up-Regulation of Insulin Receptor Expression. Metabolism 2009, 58, 109–119. [Google Scholar] [CrossRef]
- Cok, A.; Plaisier, C.; Salie, M.J.; Oram, D.S.; Chenge, J.; Louters, L.L. Berberine Acutely Activates the Glucose Transport Activity of GLUT1. Biochimie 2011, 93, 1187–1192. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.J.; Dong, H.; Li, J.-B.; Xu, L.J.; Zou, X.; Wang, K.F.; Lu, F.E.; Yi, P. Berberine Inhibits Hepatic Gluconeogenesis via the LKB1-AMPK-TORC2 Signaling Pathway in Streptozotocin-Induced Diabetic Rats. World J. Gastroenterol. 2015, 21, 7777–7785. [Google Scholar] [CrossRef]
- Dange, S.V.; Shende, S.S.; Rane, B.T.; Tilak, A.V.; Vaidya, M.U.; Limaye, M.V. An Observational Study of the Antidiabetic Activity of Berberine in Newly Diagnosed Type 2 Diabetes Mellitus Patients. J. Pharm. Biomed. Sci. 2016, 6, 5–8. [Google Scholar]
- Ming, J.; Yu, X.; Xu, X.; Wang, L.; Ding, C.; Wang, Z.; Xie, X.; Li, S.; Yang, W.; Luo, S.; et al. Effectiveness and Safety of Bifidobacterium and Berberine in Human Hyperglycemia and Their Regulatory Effect on the Gut Microbiota: A Multi-Center, Double-Blind, Randomized, Parallel-Controlled Study. Genome Med. 2021, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Hao, H.P.; Xie, H.G.; Lai, L.; Wang, Q.; Liu, C.X.; Wang, G.J. Extensive Intestinal First-Pass Elimination and Predominant Hepatic Distribution of Berberine Explain Its Low Plasma Levels in Rats. Drug Metab. Dispos. 2010, 38, 1779–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devadiga, D.; Ahipa, T.N. Betanin: A Red-Violet Pigment—Chemistry and Applications; IntechOpen: Vienna, Austria, 2020; pp. 1–19. [Google Scholar]
- Güneşer, O. Pigment and Color Stability of Beetroot Betalains in Cow Milk during Thermal Treatment. Food Chem. 2016, 196, 220–227. [Google Scholar] [CrossRef]
- Da Silva, D.V.T.; dos Baião, D.S.; de Silva, F.O.; Alves, G.; Perrone, D.; del Aguila, E.M.; Paschoalin, V.M.F. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madadi, E.; Mazloum-Ravasan, S.; Yu, J.S.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Therapeutic Application of Betalains: A Review. Plants 2020, 9, 1219. [Google Scholar] [CrossRef] [PubMed]
- Abedimanesh, N.; Asghari, S.; Mohammadnejad, K.; Daneshvar, Z.; Rahmani, S.; Shokoohi, S.; Farzaneh, A.H.; Hosseini, S.H.; Anarkooli, I.J.; Noubarani, M.; et al. The Anti-Diabetic Effects of Betanin in Streptozotocin-Induced Diabetic Rats through Modulating AMPK/SIRT1/NF-ΚB Signaling Pathway. Nutr. Metab. 2021, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Yanardaǧ, R.; Çolak, H. Effect of Chard (Beta Vulgaris L. Var. Cicla) on Blood Glucose Levels in Normal and Alloxan-Induced Diabetic Rabbits. Pharm. Pharmacol. Commun. 1998, 4, 309–311. [Google Scholar] [CrossRef]
- Bolkent, Ş.; Yanardaǧ, R.; Tabakoǧlu-Oǧuz, A.; Özsoy-Saçan, Ö. Effects of Chard (Beta Vulgaris L. Var. Cicla) Extract on Pancreatic B Cells in Streptozotocin-Diabetic Rats: A Morphological and Biochemical Study. J. Ethnopharmacol. 2000, 73, 251–259. [Google Scholar] [CrossRef]
- Clifford, T.; Constantinou, C.M.; Keane, K.M.; West, D.J.; Howatson, G.; Stevenson, E.J. The Plasma Bioavailability of Nitrate and Betanin from Beta Vulgaris Rubra in Humans. Eur. J. Nutr. 2017, 56, 1245–1254. [Google Scholar] [CrossRef] [Green Version]
- Zeece, M. Flavors. In Introduction to the Chemistry of Food; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128094341. [Google Scholar]
- Jang, M.H.; Piao, X.L.; Kim, J.M.; Kwon, S.W.; Park, J.H. Inhibition of Cholinesterase and Amyloid-β Aggregation by Resveratrol Oligomers from Vitis Amurensis. Phyther. Res. 2008, 22, 544–549. [Google Scholar] [CrossRef]
- Sen, S.; Roy, M.; Chakraborti, A.S. Ameliorative Effects of Glycyrrhizin on Streptozotocin-Induced Diabetes in Rats. J. Pharm. Pharmacol. 2011, 63, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.R.; Komarova, I.; Abdelmalak, H.D.; Yerramadha, M.R.; Ali, Y.; Ghonemi, M.; Rashad, R.; Fathy, A.; Helal, E.; Camporesi, E.M. Licorice Abuse: Time to Send a Warning Message. Ther. Adv. Endocrinol. Metab. 2012, 3, 125–138. [Google Scholar] [CrossRef]
- Takii, H.; Kometani, T.; Nishimura, T.; Nakae, T.; Okada, S.; Fushiki, T. Antidiabetic Effect of Glycyrrhizin in Genetically Diabetic KK-Ay Mice. Biol. Pharm. Bull. 2001, 24, 484–487. [Google Scholar] [CrossRef] [Green Version]
- Sil, R.; Ray, D.; Chakraborti, A.S. Glycyrrhizin Ameliorates Insulin Resistance, Hyperglycemia, Dyslipidemia and Oxidative Stress in Fructose-Induced Metabolic Syndrome-X in Rat Model. Indian J. Exp. Biol. 2013, 51, 129–138. [Google Scholar]
- Jin, S.; Fu, S.; Han, J.; Jin, S.; Lv, Q.; Lu, Y.; Qi, J.; Wu, W.; Yuan, H. Improvement of Oral Bioavailability of Glycyrrhizin by Sodium Deoxycholate/Phospholipid-Mixed Nanomicelles. J. Drug Target. 2012, 20, 615–622. [Google Scholar] [CrossRef]
- Wang, Z.; Kurosaki, Y.; Nakayama, T.; Kimura, T. Mechanism of Gastrointestinal Absorption of Glycyrrhizin in Rats. Biol. Pharm. Bull. 1994, 17, 1399–1403. [Google Scholar] [CrossRef] [Green Version]
- Baltina, L.A.; Sapozhnikova, T.A.; Gabdrakhmanova, S.F.; Makara, N.S.; Khisamutdinova, R.Y.; Baltina, L.A.; Petrova, S.F.; Saifullina, D.R.; Kondratenko, R.M. Hypoglycemic Activity of Glycyrrhizic Acid and Some of Its Derivatives in the Alloxan Diabetes Model in Rats. Pharm. Chem. J. 2021, 55, 340–344. [Google Scholar] [CrossRef]
- Renga, B.; Festa, C.; de Marino, S.; di Micco, S.; D’Auria, M.V.; Bifulco, G.; Fiorucci, S.; Zampella, A. Molecular Decodification of Gymnemic Acids from Gymnema sylvestre. Discovery of a New Class of Liver X Receptor Antagonists. Steroids 2015, 96, 121–131. [Google Scholar] [CrossRef]
- Persaud, S.J.; Al-Majed, H.; Raman, A.; Jones, P.M. Gymnema sylvestre Stimulates Insulin Release in Vitro by Increased Membrane Permeability. J. Endocrinol. 1999, 163, 207–212. [Google Scholar] [CrossRef]
- Sugihara, Y.; Nojima, H.; Matsuda, H.; Murakami, T.; Yoshikawa, M.; Kimura, I. Antihyperglycemic Effects of Gymnemic Acid IV, a Compound Derived from Gymnema sylvestre Leaves in Streptozotocin-Diabetic Mice. J. Asian Nat. Prod. Res. 2000, 2, 321–327. [Google Scholar] [CrossRef]
- Shenoy, R.S.; Prashanth, K.V.H.; Manonmani, H.K. In Vitro Antidiabetic Effects of Isolated Triterpene Glycoside Fraction from Gymnema sylvestre. Evid. Based Complement. Altern. Med. 2018, 2018, 7154702. [Google Scholar] [CrossRef] [Green Version]
- Khare, A.K.; Tondon, R.N.; Tewari, J.P. Hypoglycaemic Activity of an Indigenous Drug (Gymnema sylvestre, ’Gurmar’) in Normal and Diabetic Persons. Indian J. Physiol. Pharmacol. 1983, 27, 257–258. [Google Scholar]
- Tiwari, P.; Mishra, B.N.; Sangwan, N.S. Phytochemical and Pharmacological Properties of Gymnema sylvestre: An Important Medicinal Plant. Biomed. Res. Int. 2014, 2014, 830285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravichandran, R. Studies on Gymnemic Acids Nanoparticulate Formulations Against Diabetes Mellitus. Int. J. Biomed. Clin. Eng. 2012, 1, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Abdelrazek, H.M.A.; Kilany, O.E.; Muhammad, M.A.A.; Tag, H.M.; Abdelazim, A.M. Black Seed Thymoquinone Improved Insulin Secretion, Hepatic Glycogen Storage, and Oxidative Stress in Streptozotocin-Induced Diabetic Male Wistar Rats. Oxid. Med. Cell. Longev. 2018, 2018, 8104165. [Google Scholar] [CrossRef] [Green Version]
- Farkhondeh, T.; Samarghandian, S.; Borji, A. An Overview on Cardioprotective and Anti-Diabetic Effects of Thymoquinone. Asian Pac. J. Trop. Med. 2017, 10, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Moneim, A.A.; Abdel-Rehe, E.S.; Helmy, H.; Addaleel, W. Antidiabetic Effect of Thymoquinone via Modulation of PPAR-γ, GLUT4, Hyperlipidemia and Antioxidant Status in Diabetic Rats. Asian J. Biol. Sci. 2018, 11, 203–209. [Google Scholar] [CrossRef] [Green Version]
- El-Ameen, N.M.H.; Taha, M.M.E.; Abdelwahab, S.I.; Khalid, A.; Elfatih, F.; Kamel, M.A.; Sheikh, B.Y. Anti-Diabetic Properties of Thymoquinone Is Unassociated with Glycogen Phosphorylase Inhibition. Pharmacogn. J. 2015, 7, 406–410. [Google Scholar] [CrossRef]
- Lutfi, M.F.; Abdel-Moneim, A.M.H.; Alsharidah, A.S.; Mobark, M.A.; Abdellatif, A.A.H.; Saleem, I.Y.; Rugaie, O.A.; Mohany, K.M.; Alsharidah, M. Thymoquinone Lowers Blood Glucose and Reduces Oxidative Stress in a Rat Model of Diabetes. Molecules 2021, 26, 2348. [Google Scholar] [CrossRef]
- Karandrea, S.; Yin, H.; Liang, X.; Slitt, A.L.; Heart, E.A. Thymoquinone Ameliorates Diabetic Phenotype in Diet-Induced Obesity Mice via Activation of SIRT-1-Dependent Pathways. PLoS ONE 2017, 12, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaatabi, H.; Bamosa, A.O.; Badar, A.; Al-Elq, A.; Abou-Hozaifa, B.; Lebda, F.; Al-Khadra, A.; Al-Almaie, S. Nigella Sativa Improves Glycemic Control and Ameliorates Oxidative Stress in Patients with Type 2 Diabetes Mellitus: Placebo Controlled Participant Blinded Clinical Trial. PLoS ONE 2015, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Najmi, A.; Nasiruddin, M.; Khan, R.A.; Haque, S.F. Therapeutic Effect of Nigella Sativa in Patients of Poor Glycemic Control. Asian J. Pharm. Clin. Res. 2012, 5, 224–228. [Google Scholar]
- Thomas, J.V.; Mohan, M.E.; Prabhakaran, P.; Das, S.S.; Maliakel, B.; Krishnakumar, I.M. A Phase I Clinical Trial to Evaluate the Safety of Thymoquinone-Rich Black Cumin Oil (BlaQmax®) on Healthy Subjects: Randomized, Double-Blinded, Placebo-Controlled Prospective Study. Toxicol. Rep. 2022, 9, 999–1007. [Google Scholar] [CrossRef]
- Mohammadabadi, M.R.; Mozafari, M.R. Enhanced Efficacy and Bioavailability of Thymoquinone Using Nanoliposomal Dosage Form. J. Drug Deliv. Sci. Technol. 2018, 47, 445–453. [Google Scholar] [CrossRef]
- Rani, R.; Dahiya, S.; Dhingra, D.; Dilbaghi, N.; Kim, K.; Kumar, S. Chemico-Biological Interactions Improvement of Antihyperglycemic Activity of Nano-Thymoquinone in Rat Model of Type-2 Diabetes. Chem. Biol. Interact. 2018, 295, 119–132. [Google Scholar] [CrossRef]
- Alkharfy, K.M.; Ahmad, A.; Khan, R.M.A.; Al-Shagha, W.M. Pharmacokinetic Plasma Behaviors of Intravenous and Oral Bioavailability of Thymoquinone in a Rabbit Model. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary Sources of Lutein and Zeaxanthin Carotenoids and Their Role in Eye Health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef] [Green Version]
- Neelam, K.; Goenadi, C.J.; Lun, K.; Yip, C.C.; Eong, K.G.A. Putative Protective Role of Lutein and Zeaxanthin in Diabetic Retinopathy. Br. J. Ophthalmol. 2017, 101, 551–558. [Google Scholar] [CrossRef]
- Ozawa, Y.; Sasaki, M. Lutein and Oxidative Stress-Mediated Retinal Neurodegeneration in Diabetes; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780124058859. [Google Scholar]
- Muriach, M.; Bosch-Morell, F.; Arnal, E.; Alexander, G.; Blomhoff, R.; Romero, F.J. Lutein Prevents the Effect of High Glucose Levels on Immune System Cells in Vivo and in Vitro. J. Physiol. Biochem. 2008, 64, 149–157. [Google Scholar] [CrossRef]
- Katyal, T. Effect of Lutein in Development of Experimental Diabetic Nephropathy in Rats. Afr. J. Pharm. Pharmacol. 2013, 7, 3004–3010. [Google Scholar] [CrossRef]
- Becerra, M.O.; Contreras, L.M.; Lo, M.H.; Díaz, J.M.; Herrera, G.C. Lutein as a Functional Food Ingredient: Stability and Bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Masuzaki, H.; Kozuka, C.; Okamoto, S.; Yonamine, M.; Tanaka, H.; Shimabukuro, M. Brown Rice-Specific γ-Oryzanol as a Promising Prophylactic Avenue to Protect against Diabetes Mellitus and Obesity in Humans. J. Diabetes Investig. 2019, 10, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.W.; Ma, C.Y.; Cheng, H.H.; Chen, Y.Y.; Lai, M.H. A Rice Bran Oil Diet Improves Lipid Abnormalities and Suppress Hyperinsulinemic Responses in Rats with Streptozotocin/Nicotinamide-Induced Type 2 Diabetes. J. Clin. Biochem. Nutr. 2009, 45, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martillanes, S.; Ayuso-Yuste, M.C.; Gil, M.V.; Manzano-Durán, R.; Delgado-Adámez, J. Bioavailability, Composition and Functional Characterization of Extracts from Oryza Sativa L. Bran. Food Res. Int. 2018, 111, 299–305. [Google Scholar] [CrossRef]
- Kozuka, C.; Sunagawa, S.; Ueda, R.; Higa, M.; Tanaka, H.; Shimizu-Okabe, C.; Ishiuchi, S.; Takayama, C.; Matsushita, M.; Tsutsui, M.; et al. γ-Oryzanol Protects Pancreatic β-Cells against Endoplasmic Reticulum Stress in Male Mice. Endocrinology 2015, 156, 1242–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.M.; Chiang, P.Y. Preparation and Evaluation of Release Formulation of γ-Oryzanol/Algae Oil Self-Emulsified with Alginate Beads. Mar. Drugs 2019, 17, 156. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, Z.; Zamhuri, K.F.; Yaacob, A.; Siong, C.H.; Selvarajah, M.; Ismail, A.; Hakim, M.N. In Vitro Anti-Diabetic Activities and Chemical Analysis of Polypeptide-k and Oil Isolated from Seeds of Momordica charantia (Bitter Gourd). Molecules 2012, 17, 9631–9640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.S.; Sharathnath, K.V.; Yogeswaran, P.; Harani, A.; Sudhakar, K.; Sudha, P.; Banji, D. A Medicinal Potency of Momordica charantia. Int. J. Pharm. Sci. Rev. Res. 2010, 1, 95–100. [Google Scholar]
- Filho, M. Bitter Gourd and Pomegranate Seed Oils as Sources of α-Eleostearic and Punicic Acids: Purification and IIAntioxidant Properties in Cell Lines. Org. Med. Chem. Int. J. 2018, 5, 24–25. [Google Scholar] [CrossRef]
- Paul, D.; Dey, T.K. Comparative Prophylactic Effects of α -Eleostearic Acid Rich Nano and Conventional Emulsions in Induced Diabetic Rats. J. Food Sci. Technol. 2014, 51, 1724–1736. [Google Scholar] [CrossRef]
- Garg, V.; Kaur, J.; Singh, C.; Kaur, B.; Kumar, B.; Narang, R.; Singh, S.K. “polypeptide-k” as Phytoinsulin: How Much and How Far. Int. J. Green Pharm. 2017, 11, S197–S216. [Google Scholar]
- Lok, L.; Sirn, M.; Ahmad, M. Effects of Polypeptide-k Supplemented Soft Bun on Blood Glucose Level in Healthy Adults. Int. J. Nutr. Metab. 2011, 3, 7–10. [Google Scholar]
- Winarti, L.; Sari, L.O.R.K.; Ulfa, E.U.; Samsuri, D.A. Formulation and Antioxidant Property of Bitter Melon Seed Oil Loaded into SNEDDS as a Nutraceutical. Indones. J. Pharm. 2021, 32, 385–393. [Google Scholar] [CrossRef]
- Hamidpour, R. Medicinal Property of Sage (Saliva) for Curing Illnesses Such as Obesity, Diabetes, Depression, Dementia, Lupus, Autism, Heart Disease and Cancer: A Brief Review. Arch. Cancer Res. 2015, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Christensen, K.B.; Jørgensen, M.; Kotowska, D.; Petersen, R.K.; Kristiansen, K.; Christensen, L.P. Activation of the Nuclear Receptor PPARγ by Metabolites Isolated from Sage (Salvia officinalis L.). J. Ethnopharmacology 2010, 132, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Walch, S.G.; Tinzoh, L.N.; Zimmermann, B.F.; Stühlinger, W.; Lachenmeier, D.W. Antioxidant Capacity and Polyphenolic Composition as Quality Indicators for Aqueous Infusions of Salvia officinalis L. (Sage Tea). Front. Pharmacol. 2011, 2, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elseweidy, M.M.; Ali, A.M.A.; Elabidine, N.Z.; Mursey, N.M. Effect of Zinc Gluconate, Sage Oil on Inflammatory Patterns and Hyperglycemia in Zinc Deficient Diabetic Rats. Biomed. Pharmacother. 2017, 95, 317–323. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological Properties of Salvia officinalis and Its Components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Craft, J.; Satyal, P.; Setzer, W. The Chemotaxonomy of Common Sage (Salvia officinalis) Based on the Volatile Constituents. Medicines 2017, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, P.; Arulselvan, P.; Choi, D.K. Phytobioactive Compound-Based Nanodelivery Systems for the Treatment of Type 2 Diabetes Mellitus—Current Status. Int. J. Nanomed. 2017, 12, 1097–1111. [Google Scholar] [CrossRef] [Green Version]
- Conte, R.; de Luca, I.; de Luise, A.; Petillo, O.; Calarco, A.; Peluso, G. New Therapeutic Potentials of Nanosized Phytomedicine. J. Nanosci. Nanotechnol. 2016, 16, 8176–8187. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Ciofani, G. Nanostructured Carriers as Innovative Tools for Cancer Diagnosis and Therapy. APL Bioeng. 2019, 3, 011502. [Google Scholar] [CrossRef] [PubMed]
- Memvanga, P.B.; Nkanga, C.I. Liposomes for Malaria Management: The Evolution from 1980 to 2020. Malar. J. 2021, 20, 327. [Google Scholar] [CrossRef] [PubMed]
- Fardood, S.T.; Ramazani, A.; Moradi, S.; Asiabi, P.A. Green Synthesis of Zinc Oxide Nanoparticles Using Arabic Gum and Photocatalytic Degradation of Direct Blue 129 Dye under Visible Light. J. Mater. Sci. Mater. Electron. 2017, 28, 13596–13601. [Google Scholar] [CrossRef]
- Alomari, G.; Hamdan, S.; Al-trad, B. Gold Nanoparticles as a Promising Treatment for Diabetes and Its Complications: Current and Future Potentials According to the World Health Organization. Braz. J. Pharm. Sci. 2017, 57, 1–14. [Google Scholar]
- Čerpnjak, K.; Zvonar, A.; Gašperlin, M.; Vrečer, F. Lipid-Based Systems as a Promising Approach for Enhancing the bioavailability of pooly water-soluble drugs. Acta Pharm. 2013, 63, 427–445. [Google Scholar] [CrossRef] [Green Version]
- Nakmode, D.; Bhavana, V.; Thakor, P.; Madan, J.; Singh, P.K.; Singh, S.B.; Rosenholm, J.M.; Bansal, K.K.; Mehra, N.K. Fundamental Aspects of Lipid-Based Excipients in Lipid-Based Product Development. Pharmaceutics 2022, 14, 831. [Google Scholar] [CrossRef]
- Talegaonkar, S.; Bhattacharyya, A. Potential of Lipid Nanoparticles (SLNs and NLCs) in Enhancing Oral Bioavailability of Drugs with Poor Intestinal Permeability. AAPS PharmSciTech 2019, 20, 121. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug. Adv. NanoBiomed. Res. 2021, 2, 2100109. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Spanidi, E.; Mourtzinos, I.; Gardikis, K. Innovative Delivery Systems Loaded with Plant Bioactive Ingredients: Formulation Approaches and Applications. Plants 2021, 10, 1238. [Google Scholar] [CrossRef]
- Amjadi, S.; Abbasi, M.M.; Shokouhi, B.; Ghorbani, M.; Hamishehkar, H. Enhancement of Therapeutic Efficacy of Betanin for Diabetes Treatment by Liposomal Nanocarriers. J. Funct. Foods 2019, 59, 119–128. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, C.; Weng, W.; Bao, R.; Adu-Frimpong, M.; Toreniyazov, E.; Ji, H.; Xu, X.M.; Yu, J.N. Enhancement of Oral Bioavailability and Hypoglycemic Activity of Liquiritin-Loaded Precursor Liposome. Int. J. Pharm. 2021, 592, 120036. [Google Scholar] [CrossRef] [PubMed]
- Gauttam, V.K.; Kalia, A.N. Development of Polyherbal Antidiabetic Formulation Encapsulated in the Phospholipids Vesicle System. J. Adv. Pharm. Technol. Res. 2013, 4, 108–117. [Google Scholar] [CrossRef]
- Jose, B.; Jesy, E.J.; Nedumpara, R.J. World Journal of Pharmaceutical ReseaRch SEED EXTRACTS. World J. Pharm. Res. 2014, 3, 5041–5048. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Chen, Q.; He, Y.; Wang, H.; Yang, L.; Guo, S.; Meng, Z.; Cui, J.; Xue, M.; et al. Monodisperse Microparticles Loaded with the Self-Assembled Berberine-Phospholipid Complex-Based Phytosomes for Improving Oral Bioavailability and Enhancing Hypoglycemic Efficiency. Eur. J. Pharm. Biopharm. 2016, 103, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Rathee, S.; Kamboj, A. Optimization and Development of Antidiabetic Phytosomes by the Box–Behnken Design. J. Liposome Res. 2018, 28, 161–172. [Google Scholar] [CrossRef]
- Sharma, P.K.; Saxena, P.; Albert, J.; Chalamiah, M.; Balasubramaniam, A. Anti-Diabetic Activity of Lycopene Niosomes: Experimental Observation. J. Pharm. Drug Dev. 2017, 4, 103. [Google Scholar] [CrossRef]
- Kamble, B.; Talreja, S.; Gupta, A.; Patil, D.; Pathak, D.; Moothedath, I.; Duraiswamy, B. Development and Biological Evaluation of Gymnema sylvestre Extract-Loaded Nonionic Surfactant-Based Niosomes. Nanomedicine 2013, 8, 1295–1305. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Kouchak, M.; Badavi, M. Solid Lipid Nanoparticles of Myricitrin Have Antioxidant and Antidiabetic Effects on Streptozotocin-Nicotinamide-Induced Diabetic Model and Myotube Cell of Male Mouse. Oxid. Med. Cell. Longev. 2018, 2018, 7496936. [Google Scholar] [CrossRef] [Green Version]
- Mag, P.; Shi, F.; Wei, Z.; Zhao, Y.; Xu, X. Nanostructured Lipid Carriers Loaded with Baicalin: An Efficient Carrier for Enhanced Antidiabetic Effects. Pharmacogn. Mag. 2016, 12, 198–202. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.Y.; Liu, C.S.; Huang, C.L.; Chen, L.; Zheng, Y.R.; Huang, S.H.; Long, X.Y. Nanoemulsion Improves Hypoglycemic Efficacy of Berberine by Overcoming Its Gastrointestinal Challenge. Colloids Surf. B Biointerfaces 2019, 181, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Bachir, Y.N.; Bachir, R.N.; Hadj-ziane-zafour, A. Nanodispersions Stabilized by β-Cyclodextrin Nanosponges: Application for Simultaneous Enhancement of Bioactivity and Stability of Sage Essential Oil. Drug Dev. Ind. Pharm. 2019, 45, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Kaur, P.; Singh, S.K.; Kumar, B.; Bawa, P.; Gulati, M.; Yadav, A.K. Solid Self-Nanoemulsifying Drug Delivery Systems for Oral Delivery of Polypeptide-k: Formulation, Optimization, in-Vitro and in-Vivo Antidiabetic Evaluation. Eur. J. Pharm. Sci. 2017, 109, 297–315. [Google Scholar] [CrossRef]
- Joshi, R.P.; Negi, G.; Kumar, A.; Pawar, Y.B.; Munjal, B.; Bansal, A.K.; Sharma, S.S. SNEDDS Curcumin Formulation Leads to Enhanced Protection from Pain and Functional Deficits Associated with Diabetic Neuropathy: An Insight into Its Mechanism for Neuroprotection. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 776–785. [Google Scholar] [CrossRef]
- Balata, G.F.; Essa, E.A.; Shamardl, H.A.; Zaidan, S.H.; Abourehab, M.A.S. Self-Emulsifying Drug Delivery Systems as a Tool to Improve Solubility and Bioavailability of Resveratrol. Drug Des. Devel. Ther. 2016, 10, 117–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalpesh, C.; Ashara, K.; Paun, J.; Soniwala, M.M.; Chavada, J.R.; Nathawani, S.V.; Mori, N.; Mendapara, V.P. Vesicular Drug Delivery System: A Novel Approach. Mintage J. Pharm. Med. Sci. 2014, 3, 1–14. [Google Scholar]
- Kuntsche, J.; Horst, J.C.; Bunjes, H. Cryogenic Transmission Electron Microscopy (Cryo-TEM) for Studying the Morphology of Colloidal Drug Delivery Systems. Int. J. Pharm. 2011, 417, 120–137. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Kesharwani, P.; Gorain, B.; Low, S.Y.; Tan, S.A.; Ling, E.C.S.; Lim, Y.K.; Chin, C.M.; Lee, P.Y.; Lee, C.M.; Ooi, C.H.; et al. Nanotechnology Based Approaches for Anti-Diabetic Drugs Delivery. Diabetes Res. Clin. Pract. 2018, 136, 52–77. [Google Scholar] [CrossRef]
- Xu, Y.; Michalowski, C.B.; Beloqui, A. Advances in Lipid Carriers for Drug Delivery to the Gastrointestinal Tract. Curr. Opin. Colloid Interface Sci. 2021, 52, 101414. [Google Scholar] [CrossRef]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’agli, M.; et al. Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef] [PubMed]
- Pande, S.D.; Wagh, A.S.; Bhagure, L.B.; Patil, S.G.; Deshmukh, A.R. Preparation and Evaluation of Phytosomes of Pomegrane Peels. Res. J. Pharm. Technol. 2015, 8, 416–422. [Google Scholar] [CrossRef]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seleci, D.A.; Seleci, M.; Walter, J.G.; Stahl, F.; Scheper, T. Niosomes as Nanoparticular Drug Carriers: Fundamentals and Recent Applications. J. Nanomater. 2016, 2016, 7372306. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: A Review Emphasizing on Particle Structure and Drug Release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Xue, M.; Yang, M.; Zhang, W.; Li, X.; Gao, D.; Ou, Z.; Li, Z.; Liu, S.; Li, X.; Yang, S. Characterization, pharmacokinetics, and Hypoglycemic Effect of Berberine Loaded Solid Lipid Nanoparticles. Int. J. Nanomed. 2013, 8, 4677–4687. [Google Scholar] [CrossRef] [Green Version]
- Joshi, K.; Chandra, A.; Jain, K.; Talegaonkar, S. Nanocrystalization: An Emerging Technology to Enhance the Bioavailability of Poorly Soluble Drugs. Pharm. Nanotechnol. 2019, 7, 259–278. [Google Scholar] [CrossRef]
- Montoto, S.S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 1–24. [Google Scholar] [CrossRef]
- Liu, L.; Tang, Y.; Gao, C.; Li, Y.; Chen, S.; Xiong, T.; Li, J.; Du, M.; Gong, Z.; Chen, H.; et al. Characterization and Biodistribution in Vivo of Quercetin-Loaded Cationic Nanostructured Lipid Carriers. Colloids Surf. B Biointerfaces 2014, 115, 125–131. [Google Scholar] [CrossRef]
- Anton, N.; Vandamme, T.F. Nano-Emulsions and Micro-Emulsions: Clarifications of the Critical Differences. Pharm. Res. 2011, 28, 978–985. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An Advanced Mode of Drug Delivery System. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javadi, S.; Kazemi, N.M.; Halabian, R. Preparation of O/W Nano-Emulsion Containing Nettle and Fenugreek Extract and Cumin Essential Oil for Evaluating Antidiabetic Properties. AAPS Open 2021, 7, 13. [Google Scholar] [CrossRef]
- Djamil, R.; Zaidan, S.; Rahmat, D.; Pratami, D.K.; Hakim, F. Nanoemulsion of Okra Fruit Extract as Antidiabetic Treatment. Int. J. Appl. Pharm. 2020, 12, 138–142. [Google Scholar] [CrossRef]
- Morakul, B. Self-Nanoemulsifying Drug Delivery Systems (SNEDDS): An Advancement Technology for Oral Drug Delivery. Pharm. Sci. Asia 2020, 47, 205–220. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Kumar, B.; Wadhwa, S.; Gulati, M.; Anupriya, A.; Awasthi, A.; Vishwas, S.; Kaur, J.; Corrie, L.; et al. Self-Nanoemulsifying Composition Containing Curcumin, Quercetin, Ganoderma Lucidum Extract Powder and Probiotics for Effective Treatment of Type 2 Diabetes Mellitus in Streptozotocin Induced Rats. Int. J. Pharm. 2022, 612, 121306. [Google Scholar] [CrossRef]
- Jumaryatno, P.; Chabib, L.; Hayati, F.; Awaluddin, R. Stability Study of Ipomoea Reptans Extract Self-Nanoemulsifying Drug Delivery System (SNEDDS) as Anti-Diabetic Therapy. J. Appl. Pharm. Sci. 2018, 8, 11–14. [Google Scholar] [CrossRef] [Green Version]
- Hayati, F.; Chabib, L.; Sekarraras, F.D.; Faizah, W.S. Antihyperglycemic Activity of Centella Asiatica (L.) Urb. Leaf Ethanol Extract SNEDDS in Zebrafish (Danio Rerio). Open Chem. 2021, 19, 184–188. [Google Scholar] [CrossRef]
- Hayati, F.; Chabib, L.; Darma, D.D. Antihyperglycemia Activity of Self-Nano Emulsifying Drug-Delivery Systems (SNEDDS) of Ipomoea Reptans, Poir Leaf Ethanolic Extract in Zebrafish (Danio Rerio). AIP Conf. Proc. 2018, 2026, 020026. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green” Synthesis of Metals and Their Oxide Nanoparticles: Applications for Environmental Remediation. J. Nanobiotechnol. 2018, 16, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Khandel, P.; Yadaw, R.K.; Soni, D.K.; Kanwar, L.; Shahi, S.K. Biogenesis of Metal Nanoparticles and Their Pharmacological Applications: Present Status and Application Prospects; Springer: Berlin/Heidelberg, Germany, 2018; Volume 8. ISBN 400970180 2674.
- Ashwini, D.; Mahalingam, G. Green Synthesized Metal Nanoparticles, Characterization and Its Antidiabetic Activities-a Review. Res. J. Pharm. Technol. 2020, 13, 468–474. [Google Scholar] [CrossRef]
- Saranya, S.; Eswari, A.; Gayathri, E.; Eswari, S.; Vijayarani, K. Green Synthesis of Metallic Nanoparticles Using Aqueous Plant Extract and Their Antibacterial Activity. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1834–1845. [Google Scholar] [CrossRef]
- Kambale, E.K.; Nkanga, C.I.; Mutonkole, B.P.I.; Bapolisi, A.M.; Tassa, D.O.; Liesse, J.M.I.; Krause, R.W.M.; Memvanga, P.B. Green Synthesis of Antimicrobial Silver Nanoparticles Using Aqueous Leaf Extracts from Three Congolese Plant Species (Brillantaisia Patula, Crossopteryx Febrifuga and Senna Siamea). Heliyon 2020, 6, e04493. [Google Scholar] [CrossRef]
- Yin, J.; Hou, Y.; Yin, Y.; Song, X. Selenium-Coated Nanostructured Lipid Carriers Used for Oral Delivery of Berberine to Accomplish a Synergic Hypoglycemic Effect. Int. J. Nanomed. 2017, 12, 8671–8680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvanag, F.M.; Bayrami, A.; Habibi-Yangjeh, A.; Pouran, S.R. A Comprehensive Study on Antidiabetic and Antibacterial Activities of ZnO Nanoparticles Biosynthesized Using Silybum Marianum L Seed Extract. Mater. Sci. Eng. C 2019, 97, 397–405. [Google Scholar] [CrossRef]
- Levina, A.; Lay, P.A. Metal-Based Anti-Diabetic Drugs: Advances and Challenges. Dalt. Trans. 2011, 40, 11675–11686. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; More, P.; Nitnavare, R.; Jagtap, S.; Chippalkatti, R.; Derle, A.; Kitture, R.; Asok, A. Antidiabetic and Antioxidant Properties of Copper Nanoparticles Synthesized by Medicinal Plant Dioscorea Bulbifera. J. Nanomed. Nanotechnol. 2015, S6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.R.; Awan, F.R. Metals in the Pathogenesis of Type 2 Diabetes. J. Diabetes Metab. Disord. 2014, 13, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashrafizadeh, H.; Abtahi, S.R.; Oroojan, A.A. Trace Element Nanoparticles Improved Diabetes Mellitus; a Brief Report. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Alkaladi, A.; Abdelazim, A.M.; Afifi, M. Antidiabetic Activity of Zinc Oxide and Silver Nanoparticles on Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2014, 15, 2015–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanker, K.; Naradala, J.; Mohan, G.K.; Kumar, G.S.; Pravallika, P.L. A Sub-Acute Oral Toxicity Analysis and Comparative: In Vivo Anti-Diabetic Activity of Zinc Oxide, Cerium Oxide, Silver Nanoparticles, and Momordica charantia in Streptozotocin-Induced Diabetic Wistar Rats. RSC Adv. 2017, 7, 37158–37167. [Google Scholar] [CrossRef] [Green Version]
- Bayrami, A.; Parvinroo, S.; Habibi-Yangjeh, A.; Pouran, S.R. Bio-Extract-Mediated ZnO Nanoparticles: Microwave-Assisted Synthesis, Characterization and Antidiabetic Activity Evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 730–739. [Google Scholar] [CrossRef] [Green Version]
- Kazempour, P.; Yaghoubi, H.; Mohammadi-Aloucheh, R. Green Synthesis of ZnO Nanoparticles Using Eryngium Billardieri Leaf Extract: Characterization and Its Anti-Diabetic Properties. Biochem. Mol. Biol. 2021, 7, 18. [Google Scholar]
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Griffith, M.; Udekwu, K.I.; Gkotzis, S.; Mah, T.; Alarcon, E.I. (Eds.) Silver Nanoparticle Applications. In The Fabrication and Design of Medical and Biosensing Devices; Springer: Berlin/Heidelberg, Germany, 2015; pp. 127–146. [Google Scholar] [CrossRef]
- Balan, K.; Qing, W.; Wang, Y.; Liu, X.; Palvannan, T.; Wang, Y.; Ma, F.; Zhang, Y. Antidiabetic Activity of Silver Nanoparticles from Green Synthesis Using Lonicera Japonica Leaf Extract. RSC Adv. 2016, 6, 40162–40168. [Google Scholar] [CrossRef]
- Anbazhagan, P.; Murugan, K.; Jaganathan, A.; Sujitha, V.; Samidoss, C.M.; Jayashanthani, S.; Amuthavalli, P.; Higuchi, A.; Kumar, S.; Wei, H.; et al. Mosquitocidal, Antimalarial and Antidiabetic Potential of Musa Paradisiaca-Synthesized Silver Nanoparticles: In Vivo and In Vitro Approaches. J. Clust. Sci. 2017, 28, 91–107. [Google Scholar] [CrossRef] [Green Version]
- Sengottaiyan, A.; Aravinthan, A.; Sudhakar, C.; Selvam, K.; Srinivasan, P.; Govarthanan, M.; Manoharan, K.; Selvankumar, T. Synthesis and Characterization of Solanum Nigrum-Mediated Silver Nanoparticles and Its Protective Effect on Alloxan-Induced Diabetic Rats. J. Nanostruct. Chem. 2016, 6, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.; Pandey, P.; Sharma, P.; Shukla, A. Synthesis and Characterization of Silver Nanoparticle of Ginger Rhizome (Zingiber Officinale) Extract: Synthesis, Characterization and Anti Diabetic Activity in Streptozotocin Induced Diabetic Rats. Eur. J. Biomed. Pharm. Sci. 2016, 3, 605–610. [Google Scholar]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.K.; Rai, V.R.; Ramachandra, Y.L. Characterization, Antibacterial, Antioxidant, Antidiabetic, Anti-Inflammatory and Antityrosinase Activity of Green Synthesized Silver Nanoparticles Using Calophyllum Tomentosum Leaves Extract. Results Phys. 2018, 9, 400–408. [Google Scholar] [CrossRef]
- Campoy, A.H.G.; Gutierrez, R.M.P.; Manriquez-Alvirde, G.; Ramirez, A.M. Protection of Silver Nanoparticles Using Eysenhardtia Polystachya in Peroxide-Induced Pancreatic β-Cell Damage and Their Antidiabetic Properties in Zebrafish. Int. J. Nanomed. 2018, 13, 2601–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. In Vitro Antioxidant and Antidiabetic Activities of Zinc Oxide Nanoparticles Synthesized Using Different Plant Extracts. Bioprocess Biosyst. Eng. 2017, 40, 943–957. [Google Scholar] [CrossRef]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. RSC Advances Green Synthesis of Zinc Oxide Nanoparticles Using Hibiscus Subdari Ff a Leaf Extract. RSC Adv. 2014, 5, 4993–5003. [Google Scholar] [CrossRef]
- Vinotha, V.; Iswarya, A.; Thaya, R.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Al-Anbr, M.N.; Vaseeharan, B. Synthesis of ZnO Nanoparticles Using Insulin-Rich Leaf Extract: Anti-Diabetic, Antibiofilm and Anti-Oxidant Properties. J. Photochem. Photobiol. B Biol. 2019, 197, 111541. [Google Scholar] [CrossRef]
- Fan, D.; Li, L.; Li, Z.; Zhang, Y.; Ma, X.; Wu, L.; Zhang, H.; Guo, F. Biosynthesis of Selenium Nanoparticles and Their Protective, Antioxidative Effects in Streptozotocin Induced Diabetic Rats. Sci. Technol. Adv. Mater. 2020, 21, 505–514. [Google Scholar] [CrossRef]
- Deng, W.; Wang, H.; Wu, B.; Zhang, X. Selenium-Layered Nanoparticles Serving for Oral Delivery of Phytomedicines with Hypoglycemic Activity to Synergistically Potentiate the Antidiabetic Effect. Acta Pharm. Sin. B 2019, 9, 74–86. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, S.; Liu, Y.; Wu, W.; Shen, Y.; Zhang, L.; Li, C.; Chen, H.; Liu, A.; Shen, L.; et al. Synthesis and Antidiabetic Activity of Selenium Nanoparticles in the Presence of Polysaccharides from Catathelasma Ventricosum. Int. J. Biol. Macromol. 2018, 114, 632–639. [Google Scholar] [CrossRef]
- Badeggi, U.M.; Ismail, E.; Adeloye, A.O.; Botha, S.; Badmus, J.A.; Marnewick, J.L.; Cupido, C.N.; Hussein, A.A. Green Synthesis of Gold Nanoparticles Capped with Procyanidins from Leucosidea Sericea as Potential Antidiabetic and Antioxidant Agents. Biomolecules 2020, 10, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayakumar, S.; Vinayagam, R.; Anand, M.A.V.; Venkatachalam, K.; Saravanakumar, K.; Wang, M.H.; Sangeetha, C.C.; Gothandom, S.K.; David, E. Green Synthesis of Gold Nanoparticle Using Eclipta Alba and Its Antidiabetic Activities through Regulation of Bcl-2 Expression in Pancreatic Cell Line. J. Drug Deliv. Sci. Technol. 2020, 58, 101786. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, N.; Zhang, L.; Yin, M. Green Synthesis of Gold Nanoparticles from Fritillaria Cirrhosa and Its Anti-Diabetic Activity on Streptozotocin Induced Rats. Arab. J. Chem. 2020, 13, 5096–5106. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Govindaraju, K.; Sadiq, A.M.; Tamilselvan, S.; Kumar, V.G.; Singaravelu, G. Functionalization of Gold Nanoparticles as Antidiabetic Nanomaterial. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 116, 331–338. [Google Scholar] [CrossRef]
- Dhas, T.S.; Kumar, V.G.; Karthick, V.; Vasanth, K.; Singaravelu, G.; Govindaraju, K. Effect of Biosynthesized Gold Nanoparticles by Sargassum Swartzii in Alloxan Induced Diabetic Rats. Enzyme Microb. Technol. 2016, 95, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Daisy, P.; Saipriya, K. Biochemical Analysis of Cassia Fistula Aqueous Extract and Phytochemically Synthesized Gold Nanoparticles as Hypoglycemic Treatment for Diabetes Mellitus. Int. J. Nanomed. 2012, 7, 1189–1202. [Google Scholar] [CrossRef] [Green Version]
- Kiran, M.S.; Kumar, C.R.R.; Shwetha, U.R.; Onkarappa, H.S.; Betageri, V.S.; Latha, M.S. Green Synthesis and Characterization of Gold Nanoparticles from Moringa Oleifera Leaves and Assessment of Antioxidant, Antidiabetic and Anticancer Properties. Chem. Data Collect. 2021, 33, 100714. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Chung, I.M.; Gomathi, T.; Ansari, M.A.; Khanna, V.G.; Babu, V.; Rajakumar, G. Synthesis, Characterization and Pharmacological Potential of Green Synthesized Copper Nanoparticles. Bioprocess Biosyst. Eng. 2019, 42, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Jamdade, D.A.; Rajpali, D.; Joshi, K.A.; Kitture, R.; Kulkarni, A.S.; Shinde, V.S.; Bellare, J.; Babiya, K.R.; Ghosh, S. Gnidia Glauca- and Plumbago Zeylanica -Mediated Synthesis of Novel Copper Nanoparticles as Promising Antidiabetic Agents. Adv. Pharmacol. Sci. 2019, 2019, 9080279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faisal, S.; Jan, H.; Abdullah; Alam, I.; Rizwan, M.; Hussain, Z.; Sultana, K.; Ali, Z.; Uddin, M.N. In Vivo Analgesic, Anti-Inflammatory, and Anti-Diabetic Screening of Bacopa Monnieri-Synthesized Copper Oxide Nanoparticles. ACS Omega 2022, 7, 4071–4082. [Google Scholar] [CrossRef]
- Sasidharan, J.; Meenakshi, R.V.; Sureshkumar, P. Green Synthesis, Characterization and Evaluation of In-Vitro Antioxidant & Anti-Diabetic Activity of Nanoparticles from a Polyherbal Formulation-Mehani Green Synthesis, Characterization and Evaluation of In-Vitro Antioxidant & Anti-Diabetic Activity Of. J. Environ. Nanotechnol. 2018, 7, 51–59. [Google Scholar] [CrossRef]
- Bano, F.; Baber, M.; Ali, A.; Shah, Z.; Muhammad, S.A. Biosynthesis, Characterization, and Biological Activities of Iron Nanoparticles Using Sesamum Indicum Seeds Extract. Pharmacogn. Mag. 2017, 13, S33–S36. [Google Scholar] [CrossRef]
- Hosny, M.; Fawzy, M.; El-fakharany, E.M.; Omer, A.M.; El-monaem, E.M.A.; Khalifa, R.E.; Eltaweil, A.S. Journal of Environmental Chemical Engineering Antidiabetic, and Catalytic Applications of Platinum Nanoparticles Synthesized from Polygonum Salicifolium Leaves. J. Environ. Chem. Eng. 2022, 10, 106806. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Gu, J.; Chen, S. Biology Biosynthesis of Polyphenol-Stabilised Nanoparticles and Assessment of Anti-Diabetic Activity. J. Photochem. Photobiol. B Biol. 2017, 169, 96–100. [Google Scholar] [CrossRef]
- Surya, C.; Arul, N.A.; Pandiyan, V.; Ravikumar, S.; Amutha, P.; Sobral, A.J.F.N.; Krishnakumar, B. Costus Speciosus Leaf Extract Assisted CS-Pt-TiO 2 Composites: Synthesis, Characterization and Their Bio and Photocatalytic Applications. J. Mol. Struct. 2019, 1195, 787–795. [Google Scholar] [CrossRef]
- Samyuktha, P.S.; Ganapathy, D.M.; Rajeshkumar, S. In Vitro Study Of Antidiabetic Effect Of Green Synthesised Titanium Dioxide Nanoparticles. Nat. Volatiles Essent. Oils 2021, 8, 7260–7270. [Google Scholar]
- Langlea, A.; González-Coronel, M.A.; Carmona-Gutiérrez, G.; Moreno-Rodríguez, J.A.; Venegas, B.; Muñnoz, G.; Treviñno, S.; Díazb, A. Stevia Rebaudiana Loaded Titanium Oxide Nanomaterials as an Antidiabetic Agent in Rats. Rev. Bras. Farmacogn. 2015, 25, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Hazarika, M.; Boruah, P.K.; Pal, M.; Das, M.R.; Tamuly, C. Synthesis of Pd-RGO Nanocomposite for the Evaluation of In Vitro Anticancer and Antidiabetic Activities. ChemistrySelect 2019, 4, 1244–1250. [Google Scholar] [CrossRef]
- Shwetha, U.R.; Kumar, C.R.R.; Kiran, M.S.; Betageri, V.S.; Latha, M.S.; Veerapur, R.; Lamraoui, G.; Al-Kheraif, A.A.; Elgorban, A.M.; Syed, A.; et al. Biogenic Synthesis of Nio Nanoparticles Using Areca Catechu Leaf Extract and Their Antidiabetic and Cytotoxic Effects. Molecules 2021, 26, 2448. [Google Scholar] [CrossRef]
- Rajan, A.R.; Rajan, A.; John, A.; Philip, D. Green Synthesis of CeO 2 Nanostructures by Using Morus Nigra Fruit Extract and Its Antidiabetic Activity. AIP Conf. Proc. 2019, 2105, 020008. [Google Scholar] [CrossRef]
- Chikkanna, M.M.; Neelagund, S.E.; Rajashekarappa, K.K. Green Synthesis of Zinc Oxide Nanoparticles (ZnO NPs) and Their Biological Activity. SN Appl. Sci. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Fakhari, S.; Jamzad, M.; Fard, H.K. Green Synthesis of Zinc Oxide Nanoparticles: A Comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, F.M.; Ghasemi, N. Influence of Temperature and Concentration on Biosynthesis and Characterization of Zinc Oxide Nanoparticles Using Cherry Extract. J. Nanostruct. Chem. 2018, 8, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Ma, X.L.; Gu, Y.; Huang, H.; Zhang, G.W. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front. Chem. 2020, 8, 799. [Google Scholar] [CrossRef]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc Status Is Associated with Inflammation, Oxidative Stress, Lipid, and Glucose Metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne-Robinson, H.M.; Golden, M.H.N.; Golden, B.E.; Simeon, D.T. The Zinc Sandwich and Growth. Lancet 1991, 337, 925–926. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Otoni, C.G.; Soares, N.F.F. Zinc Oxide Nanoparticles for Food Packaging Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128007235. [Google Scholar]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A Review on Green Synthesis of Zinc Oxide Nanoparticles—An Eco-Friendly Approach. Resour. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Adibian, F.; Saddat, R.; Zahra, G.; Javid, S. Green Synthesis of Selenium Nanoparticles Using Rosmarinus Officinalis and Investigated Their Antimicrobial Activity. BioMetals 2022, 35, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Cittrarasu, V.; Kaliannan, D.; Dharman, K.; Maluventhen, V. Green Synthesis of Selenium Nanoparticles Mediated from Ceropegia Bulbosa Roxb Extract and Its Cytotoxicity, Antimicrobial, Mosquitocidal and Photocatalytic Activities. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Alagesan, V.; Venugopal, S. Green Synthesis of Selenium Nanoparticle Using Leaves Extract of Withania Somnifera and Its Biological Applications and Photocatalytic Activities. BioNanoScience 2019, 9, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Sampath, K.S.; Arunkumar, P.; Kumar, M.S.; Sujatha, V.; Premkumar, K.; Thirunavukkarasu, C. Green Synthesis and Characterization of Selenium Nanoparticles and Its Augmented Cytotoxicity with Doxorubicin on Cancer Cells. Bioprocess Biosyst. Eng. 2013, 36, 1131–1139. [Google Scholar] [CrossRef]
- Opris, R.; Tatomir, C.; Olteanu, D.; Moldovan, R.; Moldovan, B.; David, L.; Nagy, A.; Decea, N.; Kiss, M.L.; Filip, G.A. The Effect of Sambucus Nigra L. Extract and Phytosinthesized Gold Nanoparticles on Diabetic Rats. Colloids Surf. B Biointerfaces 2017, 150, 192–200. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [Green Version]
- Luzala, M.M.; Muanga, C.K.; Kyana, J.; Safari, J.B.; Zola, E.N.; Mbusa, G.V.; Nuapia, Y.B.; Liesse, J.-M.I.; Nkanga, C.I.; Krause, R.W.M.; et al. A Critical Review of the Antimicrobial and Antibiofilm Activities of Green-Synthesized Plant-Based Metallic Nanoparticles. Nanomaterials 2022, 12, 1841. [Google Scholar] [CrossRef]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.M. Green Approach for Synthesis of Zinc Oxide Nanoparticles from Andrographis Paniculata Leaf Extract and Evaluation of Their Antioxidant, Anti-Diabetic, and Anti-Inflammatory Activities. Bioprocess Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Green Synthesis of Metallic Nanoparticles from Natural Resources and Food Waste and Their Environmental Application; Kanchi, S., Ahmed, S., Eds.; John and Wiley and Sons: Hoboken, NJ, USA, 2018; pp. 321–385. [Google Scholar] [CrossRef]
- Altaf, M.; Manoharadas, S.; Zeyad, M.T. Green Synthesis of Cerium Oxide Nanoparticles Using Acorus Calamus Extract and Their Antibiofilm Activity against Bacterial Pathogens. Microsc. Res. Tech. 2021, 84, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Muthuvel, A.; Jothibas, M.; Mohana, V.; Manoharan, C. Green Synthesis of Cerium Oxide Nanoparticles Using Calotropis Procera Flower Extract and Their Photocatalytic Degradation and Antibacterial Activity. Inorg. Chem. Commun. 2020, 119, 108086. [Google Scholar] [CrossRef]
- Khan, M.; Mashwani, Z.-R.; Ikram, M.; Raja, N.I.; Mohamed, A.H.; Ren, G.; Omar, A.A. Efficacy of Green Cerium Oxide Nanoparticles for Potential Therapeutic Applications: Circumstantial Insight on Mechanistic Aspects. Nanomaterials 2022, 12, 2117. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Salih, E. Green Synthesis of Metallic Nanoparticles Using Biopolymers and Plant Extracts; Kanshi, S., Ahmed, S., Eds.; John and Wiley and Sons: Hoboken, NJ, USA, 2018; pp. 293–319. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kambale, E.K.; Quetin-Leclercq, J.; Memvanga, P.B.; Beloqui, A. An Overview of Herbal-Based Antidiabetic Drug Delivery Systems: Focus on Lipid- and Inorganic-Based Nanoformulations. Pharmaceutics 2022, 14, 2135. https://doi.org/10.3390/pharmaceutics14102135
Kambale EK, Quetin-Leclercq J, Memvanga PB, Beloqui A. An Overview of Herbal-Based Antidiabetic Drug Delivery Systems: Focus on Lipid- and Inorganic-Based Nanoformulations. Pharmaceutics. 2022; 14(10):2135. https://doi.org/10.3390/pharmaceutics14102135
Chicago/Turabian StyleKambale, Espoir K., Joëlle Quetin-Leclercq, Patrick B. Memvanga, and Ana Beloqui. 2022. "An Overview of Herbal-Based Antidiabetic Drug Delivery Systems: Focus on Lipid- and Inorganic-Based Nanoformulations" Pharmaceutics 14, no. 10: 2135. https://doi.org/10.3390/pharmaceutics14102135