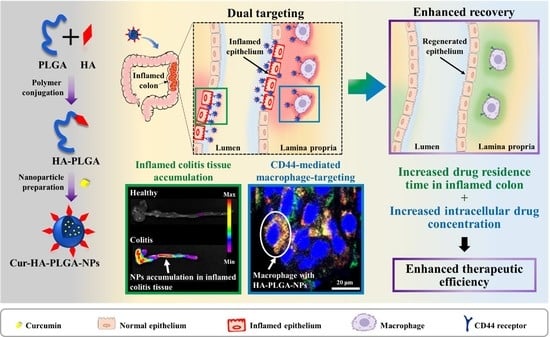
Hyaluronic Acid-Conjugated PLGA Nanoparticles Alleviate Ulcerative Colitis via CD44-Mediated Dual Targeting to Inflamed Colitis Tissue and Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of HA-Conjugated PLGA Polymer (HA-PLGA)
2.3. Preparation of NPs
2.4. Characterization of HA-conjugated PLGA Polymer (HA-PLGA)
2.5. Morphology of NPs
2.6. Size and Size Distribution Analysis of NPs
2.7. Determination of Loading Capacity (%), Encapsulation Efficiency (%), and In Vitro Release Pattern
2.8. Animal Studies
2.8.1. Induction of DSS-Induced Colitis in Mice
2.8.2. In Vivo Tissue Accumulation of NPs on Healthy and Inflamed Colitis Tissue of Mice
2.8.3. In Vivo NP Uptake by Activated Macrophages of Inflamed Colitis Tissue
2.8.4. Evaluation of Colitis Severity via DAI and Survival Rate
2.8.5. Evaluation of Colon Length, Colon Weight/Length Ratio, and Spleen Weight
2.8.6. Therapeutic Efficacy in a DSS-induced Colitis Mice Model
2.8.7. Histological Assessment of Colitis
2.8.8. Infiltration of Macrophages in Colitis Tissue
2.8.9. Quantification of Pro-inflammatory Cytokines via ELISA
2.8.10. Statistical Analysis
3. Results and Discussion
3.1. HA-PLGA Polymer Characterization
3.2. Fabrication and Characterization of PLGA-HA NPs
3.3. In Vivo Imaging of Accumulation of NPs on the Healthy and Inflamed Mice Colon
3.4. In Vivo CLSM Imaging of Cellular Uptake of NPs by Inflammatory Macrophages
3.5. Macroscopic Analysis of Colitis after Treatment with NPs
3.6. Histological Analysis and Immunofluorescence of Colitis after Treatment with NPs
3.7. Measurement of Pro-inflammatory Cytokine Levels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kathleen, A.; Head, N.D.; Julie, S.; Jurenka, M. Inflammatory bowel disease part i: Ulcerative colitis–pathophysiology and conventional and alternative treatment options. Altern. Med. Rev. 2003, 8, 247–283. [Google Scholar]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandborn, W.J.; Targan, S.R. Biologic therapy of inflammatory bowel disease. Gastroenterology 2002, 122, 1592–1608. [Google Scholar] [CrossRef] [PubMed]
- Kornbluth, A.; Sachar, D.B. Ulcerative Colitis Practice Guidelines in Adults: American College of Gastroenterology, Practice Parameters Committee. Am. J. Gastroenterol. 2010, 105, 501–523. [Google Scholar] [CrossRef]
- Feagan, B.G.; Chande, N.; Macdonald, J.K. Are There Any Differences in the Efficacy and Safety of Different Formulations of Oral 5-ASA Used for Induction and Maintenance of Remission in Ulcerative Colitis? Evidence from Cochrane Reviews. Inflamm. Bowel Dis. 2013, 19, 2031–2040. [Google Scholar] [CrossRef]
- Kato, S.; Ishibashi, A.; Kani, K.; Yakabi, K. Optimized Management of Ulcerative Proctitis: When and How to Use Mesalazine Suppository. Digestion 2018, 97, 59–63. [Google Scholar] [CrossRef]
- Meijer, B.; Mulder, C.J.J.; Bouma, G.; Ponsioen, C.Y.; Van Der Woude, C.J.; Van Der Meulen, A.E.; Wintjens, D.S.J.; Dijkstra, G.; Hoentjen, F.; Oldenburg, B.; et al. Methotrexate and Thioguanine Rescue Therapy for Conventional Thiopurine Failing Ulcerative Colitis Patients: A Multi-center Database Study on Tolerability and Effectiveness. Inflamm. Bowel Dis. 2018, 24, 1558–1565. [Google Scholar] [CrossRef] [Green Version]
- Lamprecht, A.; Schäfer, U.; Lehr, C.-M.M. Size-Dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001, 18, 788–793. [Google Scholar] [CrossRef]
- Oshi, M.A.; Lee, J.; Naeem, M.; Hasan, N.; Kim, J.; Kim, H.J.; Lee, E.H.; Jung, Y.; Yoo, J.-W. Curcumin Nanocrystal/pH-Responsive Polyelectrolyte Multilayer Core–Shell Nanoparticles for Inflammation-Targeted Alleviation of Ulcerative Colitis. Biomacromolecules 2020, 21, 3571–3581. [Google Scholar] [CrossRef]
- Oshi, M.A.; Naeem, M.; Bae, J.; Kim, J.; Lee, J.; Hasan, N.; Kim, W.; Im, E.; Jung, Y.; Yoo, J.-W. Colon-targeted dexamethasone microcrystals with pH-sensitive chitosan/alginate/Eudragit S multilayers for the treatment of inflammatory bowel disease. Carbohydr. Polym. 2018, 198, 434–442. [Google Scholar] [CrossRef]
- Johansson, M.E.V. Mucus Layers in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2014, 20, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Y.; Huang, Y.; Ma, Y.; Lv, J.; Xiao, B. Mucus-penetrating polymeric nanoparticles for oral delivery of curcumin to inflamed colon tissue. J. Drug Deliv. Sci. Technol. 2019, 52, 157–164. [Google Scholar] [CrossRef]
- Bakhtiar, A.; Liew, Q.X.; Ng, K.Y.; Chowdhury, E.H. Active targeting via ligand-anchored pH-responsive strontium nanoparticles for efficient nucleic acid delivery into breast cancer cells. J. Pharm. Investig. 2022, 52, 243–257. [Google Scholar] [CrossRef]
- He, W.; Kapate, N.; Shields, C.W.; Mitragotri, S. Drug delivery to macrophages: A review of targeting drugs and drug carriers to macrophages for inflammatory diseases. Adv. Drug Deliv. Rev. 2020, 165–166, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Farkas, S.; Hornung, M.; Sattler, C.; Anthuber, M.; Gunthert, U.; Herfarth, H.; Schlitt, H.J.; Geissler, E.K.; Wittig, B.M. Short-term treatment with anti-CD44v7 antibody, but not CD44v4, restores the gut mucosa in established chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin. Exp. Immunol. 2005, 142, 260–267. [Google Scholar] [CrossRef]
- Hankard, F.; Cezard, J.P.; Aigrain, Y.; Navarro, J.; Peuchmaur, M. CD44 variant expression in inflammatory colonic mucosa is not disease specific but associated with increased crypt cell proliferation. Histopathology 1998, 32, 317–321. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Morton, S.W.; Shopsowitz, K.E.; Choi, J.-H.; Deng, Z.J.; Cho, N.-J.; Hammond, P.T. Bimodal Tumor-Targeting from Microenvironment Responsive Hyaluronan Layer-by-Layer (LbL) Nanoparticles. ACS Nano 2014, 8, 8374–8382. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, W.; Jackson, D.; Trowell, J.; Bell, J.; Prince, C.; Chapman, R.; Jewel, D.; Kaklamanis, L.; Fox, S.; Simmons, D. Increased expression of CD44v6 and CD44v3 in ulcerative colitis but not colonic Crohn’s disease. Lancet 1995, 345, 1205–1209. [Google Scholar] [CrossRef]
- Mahida, Y.R. The Key Role of Macrophages in the Immunopathogenesis of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2000, 6, 21–33. [Google Scholar] [CrossRef]
- Cai, X.; Wang, X.; He, M.; Wang, Y.; Lan, M.; Zhao, Y.; Gao, F. Colon-targeted delivery of tacrolimus using pH-responsive polymeric nanoparticles for murine colitis therapy. Int. J. Pharm. 2021, 606, 120836. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, C.; Liu, D.; Han, M.K.; Wang, L.; Merlin, D. Oral Delivery of Nanoparticles Loaded With Ginger Active Compound, 6-Shogaol, Attenuates Ulcerative Colitis and Promotes Wound Healing in a Murine Model of Ulcerative Colitis. J. Crohn’s Colitis 2018, 12, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Poh, S.; Chelvam, V.; Ayala-López, W.; Putt, K.S.; Low, P.S. Selective liposome targeting of folate receptor positive immune cells in inflammatory diseases. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; He, S.; Cui, S.; Shi, Y.; Tan, Y.; Li, Z.; Huang, C.; Liu, D.; Zhi, F.; Peng, L. A Molecular Targeted Immunotherapeutic Strategy for Ulcerative Colitis via Dual-targeting Nanoparticles Delivering miR-146b to Intestinal Macrophages. J. Crohn’s Colitis 2019, 13, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gan, J.; Jia, L.; Guo, G.; Wang, C.; Zang, Y.; Ding, Z.; Chen, J.; Zhang, J.; Dong, L. An orally administrated nucleotide-delivery vehicle targeting colonic macrophages for the treatment of inflammatory bowel disease. Biomaterials 2015, 48, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.; Napit, P.; Briski, K.; Mattheolabakis, G. Oral Delivery of Nucleic Acids with Passive and Active Targeting to the Intestinal Tissue Using Polymer-Based Nanocarriers. Pharmaceutics 2021, 13, 1075. [Google Scholar] [CrossRef]
- Liu, P.; Gao, C.; Chen, H.; Vong, C.T.; Wu, X.; Tang, X.; Wang, S.; Wang, Y. Receptor-mediated targeted drug delivery systems for treatment of inflammatory bowel disease: Opportunities and emerging strategies. Acta Pharm. Sin. B 2021, 11, 2798–2818. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Merlin, D. Homeostatic and innate immune responses: Role of the transmembrane glycoprotein CD98. Experientia 2012, 69, 3015–3026. [Google Scholar] [CrossRef] [Green Version]
- Kucharzik, T.; Lugering, A.; Yan, Y.; Driss, A.; Charrier, L.; Sitaraman, S.; Merlin, D. Activation of epithelial CD98 glycoprotein perpetuates colonic inflammation. Lab. Investig. 2005, 85, 932–941. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Naeem, M.; Noh, J.-K.; Lee, E.H.; Yoo, J.-W. Dexamethasone phosphate-loaded folate-conjugated polymeric nanoparticles for selective delivery to activated macrophages and suppression of inflammatory responses. Macromol. Res. 2015, 23, 485–492. [Google Scholar] [CrossRef]
- Gou, S.; Huang, Y.; Wan, Y.; Ma, Y.; Zhou, X.; Tong, X.; Huang, J.; Kang, Y.; Pan, G.; Dai, F.; et al. Multi-bioresponsive silk fibroin-based nanoparticles with on-demand cytoplasmic drug release capacity for CD44-targeted alleviation of ulcerative colitis. Biomaterials 2019, 212, 39–54. [Google Scholar] [CrossRef]
- Zhu, S.; Niu, M.; O’Mary, H.; Cui, Z. Targeting of Tumor-Associated Macrophages Made Possible by PEG-Sheddable, Mannose-Modified Nanoparticles. Mol. Pharm. 2013, 10, 3525–3530. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.; Lindsay, J.O.; Sturm, A.; Windsor, A.; Colombel, J.-F.; Allez, M.; D’Haens, G.; D’Hoore, A.; Mantzaris, G.; Novacek, G.; et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis Part 2: Current management. J. Crohn’s Colitis 2012, 6, 991–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.-H.; Jones, S.A.; Forbes, B.; Martin, G.P.; Brown, M.B. Hyaluronan: Pharmaceutical Characterization and Drug Delivery. Drug Deliv. 2005, 12, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Liu, Y.; Ren, Y.; Wang, J.; Niu, B.; Li, W. Preparation of curcumin loaded hyaluronic acid-poly (lactic-co-glycolic acid) micelles with pH response and tumor targeting. Eur. Polym. J. 2022, 177, 111450. [Google Scholar] [CrossRef]
- Lu, H.-D.; Zhao, H.-Q.; Wang, K.; Lv, L.-L. Novel hyaluronic acid–chitosan nanoparticles as non-viral gene delivery vectors targeting osteoarthritis. Int. J. Pharm. 2011, 420, 358–365. [Google Scholar] [CrossRef]
- Jana, P.; Shyam, M.; Singh, S.; Jayaprakash, V.; Dev, A. Biodegradable polymers in drug delivery and oral vaccination. Eur. Polym. J. 2020, 142, 110155. [Google Scholar] [CrossRef]
- Anwar, M.; Muhammad, F.; Akhtar, B. Biodegradable nanoparticles as drug delivery devices. J. Drug Deliv. Sci. Technol. 2021, 64, 102638. [Google Scholar] [CrossRef]
- Jacob, A.; Wu, R.; Zhou, M.; Wang, P. Mechanism of the Anti-inflammatory Effect of Curcumin: PPAR-γActivation. PPAR Res. 2007, 2007, 89369. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Hlaing, S.P.; Hasan, N.; Kwak, D.; Kim, H.; Cao, J.; Yoon, I.-S.; Yun, H.; Jung, Y.; Yoo, J.-W. Tumor-Penetrable Nitric Oxide-Releasing Nanoparticles Potentiate Local Antimelanoma Therapy. ACS Appl. Mater. Interfaces 2021, 13, 30383–30396. [Google Scholar] [CrossRef]
- Hlaing, S.P.; Kim, J.; Lee, J.; Hasan, N.; Cao, J.; Naeem, M.; Lee, E.H.; Shin, J.H.; Jung, Y.; Lee, B.-L.; et al. S-Nitrosoglutathione loaded poly(lactic-co-glycolic acid) microparticles for prolonged nitric oxide release and enhanced healing of methicillin-resistant Staphylococcus aureus-infected wounds. Eur. J. Pharm. Biopharm. 2018, 132, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef] [Green Version]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. A J. Tech. Methods Pathol. 1993, 69, 238–249. Available online: https://europepmc.org/article/med/99#impact (accessed on 1 August 2022).
- Makhlof, A.; Tozuka, Y.; Takeuchi, H. pH-Sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model. Eur. J. Pharm. Biopharm. 2009, 72, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cummins, E.P.; Seeballuck, F.; Keely, S.J.; Mangan, N.E.; Callanan, J.J.; Fallon, P.G.; Taylor, C.T. The Hydroxylase Inhibitor Dimethyloxalylglycine Is Protective in a Murine Model of Colitis. Gastroenterology 2008, 134, 156–165.e1. [Google Scholar] [CrossRef] [PubMed]
- Weigmann, B.; Lehr, H.A.; Yancopoulos, G.; Valenzuela, D.; Murphy, A.; Stevens, S.; Schmidt, J.; Galle, P.R.; Rose-John, S.; Neurath, M.F. The transcription factor NFATc2 controls IL-6–dependent T cell activation in experimental colitis. J. Exp. Med. 2008, 205, 2099–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, N.A.; Bruss, M.S.; Gardner, M.; Willis, W.L.; Mo, X.; Valiente, G.R.; Cao, Y.; Liu, Z.; Jarjour, W.N.; Wu, L.-C. Oral Administration of Nano-Emulsion Curcumin in Mice Suppresses Inflammatory-Induced NFκB Signaling and Macrophage Migration. PLoS ONE 2014, 9, e111559. [Google Scholar] [CrossRef]
- Ukil, A.; Maity, S.; Karmakar, S.; Datta, N.; Vedasiromoni, J.R.; Das, P.K. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. J. Cereb. Blood Flow Metab. 2003, 139, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Lertpairod, J.; Tiyaboonchai, W. pH-sensitive beads containing curcumin loaded nanostructured lipid carriers for a colon targeted oral delivery system. J. Pharm. Investig. 2022, 52, 387–396. [Google Scholar] [CrossRef]
- Price, L.C.; Buescher, R. Kinetics of Alkaline Degradation of the Food Pigments Curcumin and Curcuminoids. J. Food Sci. 1997, 62, 267–269. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Másson, M.; Loftsson, T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: Solubility, chemical and photochemical stability. Int. J. Pharm. 2002, 244, 127–135. [Google Scholar] [CrossRef]
- Van Der Waaij, L.A.; Harmsen, H.J.M.; Madjipour, M.; Kroese, F.G.M.; Zwiers, M.; Van Dullemen, H.M.; De Boer, N.K.; Welling, G.W.; Jansen, P.L.M. Bacterial Population Analysis of Human Colon and Terminal Ileum Biopsies with 16S rRNA-based Fluorescent Probes: Commensal Bacteria Live in Suspension and Have No Direct Contact with Epithelial Cells. Inflamm. Bowel Dis. 2005, 11, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, S.; Zhu, C.; Zhu, Q.; Gan, Y.; Rantanen, J.; Rahbek, U.L.; Hovgaard, L.; Yang, M. Intestinal mucosa permeability following oral insulin delivery using core shell corona nanolipoparticles. Biomaterials 2013, 34, 9678–9687. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shan, W.; Zhang, Z.; Huang, Y. Engineering nanomaterials to overcome the mucosal barrier by modulating surface properties. Adv. Drug Deliv. Rev. 2018, 124, 150–163. [Google Scholar] [CrossRef]
- Johansson, M.E.V. Fast Renewal of the Distal Colonic Mucus Layers by the Surface Goblet Cells as Measured by In Vivo Labeling of Mucin Glycoproteins. PLoS ONE 2012, 7, e41009. [Google Scholar] [CrossRef]
- Xiao, B.; Xu, Z.; Viennois, E.; Zhang, Y.; Zhang, Z.; Zhang, M.; Han, M.K.; Kang, Y.; Merlin, D. Orally Targeted Delivery of Tripeptide KPV via Hyaluronic Acid-Functionalized Nanoparticles Efficiently Alleviates Ulcerative Colitis. Mol. Ther. 2017, 25, 1628–1640. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.-H.; Rastogi, R.; Shelke, J.; Amiji, M.M. Modulation of Macrophage Functional Polarity towards Anti-Inflammatory Phenotype with Plasmid DNA Delivery in CD44 Targeting Hyaluronic Acid Nanoparticles. Sci. Rep. 2015, 5, 16632. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Shajib, S.; Manocha, M.M.; Khan, W.I. Investigating Intestinal Inflammation in DSS-induced Model of IBD. J. Vis. Exp. 2012, 60, 3678. [Google Scholar] [CrossRef] [Green Version]
- Viennois, E.; Xiao, B.; Ayyadurai, S.; Wang, L.; Wang, P.G.; Zhang, Q.; Chen, Y.; Merlin, D. Micheliolide, a new sesquiterpene lactone that inhibits intestinal inflammation and colitis-associated cancer. Lab. Investig. 2014, 94, 950–965. [Google Scholar] [CrossRef] [Green Version]
- Poh, S.; Lin, J.B.; Panitch, A. Release of Anti-inflammatory Peptides from Thermosensitive Nanoparticles with Degradable Cross-Links Suppresses Pro-inflammatory Cytokine Production. Biomacromolecules 2015, 16, 1191–1200. [Google Scholar] [CrossRef] [Green Version]
- Uings, I.; Puxeddu, I.; Temkin, V.; Smith, S.J.; Fattah, D.; Ray, K.P.; Levi-Schaffer, F. Effects of dexamethasone on TNF-alpha-induced release of cytokines from purified human blood eosinophils. Clin. Mol. Allergy 2005, 3, 5. [Google Scholar] [CrossRef] [PubMed]







| Formulation | Size (nm) | Polydispersity Index | Loading Capacity (%) | Encapsulation Efficiency (%) | Zeta Potential (mV) |
|---|---|---|---|---|---|
| Cur-PLGA-NPs | 225.0 ± 3.55 | 0.03 ± 0.02 | 5.3 ± 0.3 | 58.3 ± 3.7 | −13.6 ± 0.3 |
| Cur-HA-PLGA-NPs | 234.4 ± 1.27 | 0.02 ± 0.01 | 5.8 ± 0.3 | 63.4 ± 3.6 | −14.6 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlaing, S.P.; Cao, J.; Lee, J.; Kim, J.; Saparbayeva, A.; Kwak, D.; Kim, H.; Hwang, S.; Yun, H.; Moon, H.R.; et al. Hyaluronic Acid-Conjugated PLGA Nanoparticles Alleviate Ulcerative Colitis via CD44-Mediated Dual Targeting to Inflamed Colitis Tissue and Macrophages. Pharmaceutics 2022, 14, 2118. https://doi.org/10.3390/pharmaceutics14102118
Hlaing SP, Cao J, Lee J, Kim J, Saparbayeva A, Kwak D, Kim H, Hwang S, Yun H, Moon HR, et al. Hyaluronic Acid-Conjugated PLGA Nanoparticles Alleviate Ulcerative Colitis via CD44-Mediated Dual Targeting to Inflamed Colitis Tissue and Macrophages. Pharmaceutics. 2022; 14(10):2118. https://doi.org/10.3390/pharmaceutics14102118
Chicago/Turabian StyleHlaing, Shwe Phyu, Jiafu Cao, Juho Lee, Jihyun Kim, Aruzhan Saparbayeva, Dongmin Kwak, Hyunwoo Kim, Seonghwan Hwang, Hwayoung Yun, Hyung Ryong Moon, and et al. 2022. "Hyaluronic Acid-Conjugated PLGA Nanoparticles Alleviate Ulcerative Colitis via CD44-Mediated Dual Targeting to Inflamed Colitis Tissue and Macrophages" Pharmaceutics 14, no. 10: 2118. https://doi.org/10.3390/pharmaceutics14102118