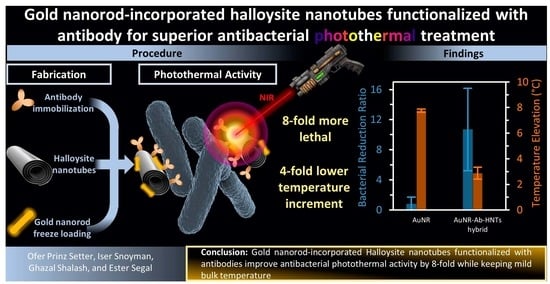
Gold Nanorod-Incorporated Halloysite Nanotubes Functionalized with Antibody for Superior Antibacterial Photothermal Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Functionalization of HNTs with Anti-E. coli Antibody
2.3. AuNR-Ab-HNTs Hybrid Preparation
2.4. Infrared Spectroscopy
2.5. Fluorescence Immunolabeling
2.6. Transmission Electron Microscopy and Energy Dispersive X-ray Spectroscopy (EDX)
2.7. Cytotoxicity
2.8. Antibacterial Photothermal Treatment (APTT)
2.8.1. Particle and Bacteria Mixture Preparation
2.8.2. Near Infrared Irradiation
2.9. Plate Count
2.10. Live/Dead Cell Staining
2.11. Scanning Electron Microscopy
3. Results and Discussion
3.1. Fabrication of AuNR-Ab-HNTs Hybrids
3.2. Cytotoxicity
3.3. Antibacterial Photothermal Treatment (APTT)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Wilkins, L.J.; Monga, M.; Miller, A.W. Colonocyte Metabolism Plays an Essential Role in Balancing the Gut Microbiota. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M.A.T. Antibiotics Special Issue: Challenges and Opportunities in Antibiotic Discovery and Development. ACS Infect. Dis. 2020, 6, 1286–1288. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [PubMed]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A New Paradigm for Treating Infectious Diseases Using Nanomaterials in the Antibiotics Resistant Era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-Based Therapeutics for Antibiotic-Resistant Bacterial Infections. Nat. Rev. Microbiol. 2020, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Okkeh, M.; Bloise, N.; Restivo, E.; de Vita, L.; Pallavicini, P.; Visai, L. Gold Nanoparticles: Can They Be the next Magic Bullet for Multidrug-Resistant Bacteria? Nanomaterials 2021, 11, 312. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The Golden Age: Gold Nanoparticles for Biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, L.; Zhang, J.; Aslan, H.; Dong, M. Photoactive Antimicrobial Nanomaterials. J. Mater. Chem. B 2017, 5, 8631–8652. [Google Scholar] [CrossRef]
- Norman, R.S.; Stone, J.W.; Gole, A.; Murphy, C.J.; Sabo-Attwood, T.L. Targeted Photothermal Lysis of the Pathogenic Bacteria, Pseudomonas aeruginosa, with Gold Nanorods. Nano Lett. 2008, 8, 302–306. [Google Scholar] [CrossRef]
- Qiang, L.; Jin, H.; Feng, Y.; Wu, R.; Song, Y.; Liu, L. Apoptosis-like Bacterial Death Modulated by Photoactive Hyperthermia Nanomaterials and Enhanced Wound Disinfection Application. Nanoscale 2021, 13, 14785–14794. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Jiménez, C.; Romney, M.G.; Roa-Flores, S.A.; Martínez-Castañón, G.; Bach, H. Hydrogel-Embedded Gold Nanorods Activated by Plasmonic Photothermy with Potent Antimicrobial Activity. Nanomedicine 2019, 22, 102093. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, N.; Han, L.; Chen, M.L.; Wang, J.H. Core–Shell–Shell Nanorods for Controlled Release of Silver That Can Serve as a Nanoheater for Photothermal Treatment on Bacteria. Acta Biomater. 2015, 11, 511–519. [Google Scholar] [CrossRef]
- Spinks, A.T.; Dunstan, R.H.; Harrison, T.; Coombes, P.; Kuczera, G. Thermal Inactivation of Water-Borne Pathogenic and Indicator Bacteria at Sub-Boiling Temperatures. Water Res. 2006, 40, 1326–1332. [Google Scholar] [CrossRef]
- Blackburn, C.D.W.; Curtis, L.M.; Humpheson, L.; Billon, C.; McClure, P.J. Development of Thermal Inactivation Models for Salmonella Enteritidis and Escherichia Coli O157:H7 with Temperature, PH and NaCl as Controlling Factors. Int. J. Food Microbiol. 1997, 38, 31–44. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Thompson, L.B.; Boulos, S.P.; Sisco, P.N.; Murphy, C.J. Gold Nanorods: Their Potential for Photothermal Therapeutics and Drug Delivery, Tempered by the Complexity of Their Biological Interactions. Adv. Drug Deliv. Rev. 2012, 64, 190–199. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of Gold Nanoparticles (AuNPs): A Review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef] [PubMed]
- Pamies, R.; Cifre, J.G.H.; Espín, V.F.; Collado-González, M.; Baños, F.G.D.; de La Torre, J.G. Aggregation Behaviour of Gold Nanoparticles in Saline Aqueous Media. J. Nanoparticle Res. 2014, 16, 2376. [Google Scholar] [CrossRef]
- Stavitskaya, A.; Khusnetdenova, E.; Vinokurov, V.; Lvov, Y.; Fakhrullin, R. Prokaryotic and Eukaryotic Toxicity of Halloysite Decorated with Photoactive Nanoparticles. Chem. Commun. 2022, 58, 7719–7729. [Google Scholar] [CrossRef]
- Kornilova, A.; Novikov, S.; Kuralbayeva, G.; Jana, S.; Lysenko, I.; Shpichka, A.; Stavitskaya, A.; Gorbachevskii, M.; Novikov, A.; Ikramova, S.; et al. Halloysite Nanotubes with Immobilized Plasmonic Nanoparticles for Biophotonic Applications. Appl. Sci. 2021, 11, 4565. [Google Scholar] [CrossRef]
- Joussein, E. Geology and Mineralogy of Nanosized Tubular Halloysite. In Developments in Clay Science; Peng Yuan, A.T., Faïza, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 7, pp. 12–48. ISBN 1572-4352. [Google Scholar]
- Prinz Setter, O.; Segal, E. Halloysite Nanotubes—The Nano-Bio Interface. Nanoscale 2020, 12, 23444–23460. [Google Scholar] [CrossRef] [PubMed]
- Rozhina, E.; Batasheva, S.; Miftakhova, R.; Yan, X.; Vikulina, A.; Volodkin, D.; Fakhrullin, R. Comparative Cytotoxicity of Kaolinite, Halloysite, Multiwalled Carbon Nanotubes and Graphene Oxide. Appl. Clay Sci. 2021, 205, 106041. [Google Scholar] [CrossRef]
- Awad, M.E.; Lopez-Galindo, A.; Setti, M.; El-Rahmany, M.M.; Iborra, C.V. Kaolinite in Pharmaceutics and Biomedicine. Int. J. Pharm. 2017, 533, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.R.; Shoaib, M.H.; Yousuf, R.I.; Ali, T.; Geckeler, K.E.; Siddiqui, F.; Ahmed, K.; Qazi, F. Clay Nanotubes as a Novel Multifunctional Excipient for the Development of Directly Compressible Diclofenac Potassium Tablets in a SeDeM Driven QbD Environment. Eur. J. Pharm. Sci. 2019, 133, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite Clay Nanotubes for Loading and Sustained Release of Functional Compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Ferreira, C.; Veiga, F.; Ribeiro, A.J.; Panchal, A.; Lvov, Y.; Agarwal, A. Halloysite Clay Nanotubes for Life Sciences Applications: From Drug Encapsulation to Bioscaffold. Adv. Colloid Interface Sci. 2018, 257, 58–70. [Google Scholar] [CrossRef]
- Satish, S.; Tharmavaram, M.; Rawtani, D. Halloysite Nanotubes as a Nature’s Boon for Biomedical Applications. Nanobiomedicine 2019, 6, 1–16. [Google Scholar] [CrossRef]
- Prinz Setter, O.; Dahan, L.; Abu Hamad, H.; Segal, E. Acid-Etched Halloysite Nanotubes as Superior Carriers for Ciprofloxacin. Appl. Clay Sci. 2022, 228, 106629. [Google Scholar] [CrossRef]
- Gorbachevskii, M.; Stavitskaya, A.; Novikov, A.; Fakhrullin, R.; Rozhina, E.; Naumenko, E.; Vinokurov, V. Fluorescent Gold Nanoclusters Stabilized on Halloysite Nanotubes: In Vitro Study on Cytotoxicity. Appl. Clay Sci. 2021, 207, 106106. [Google Scholar] [CrossRef]
- Kornilova, A.; Gorbachevskii, M.; Kuralbayeva, G.; Jana, S.; Novikov, A.; Eliseev, A.; Vasiliev, A.; Timoshenko, V.Y. Plasmonic Properties of Halloysite Nanotubes with Immobilized Silver Nanoparticles for Applications in Surface-Enhanced Raman Scattering. Phys. Status Solidi (a) 2019, 216, 1800886. [Google Scholar] [CrossRef]
- Karewicz, A.; Machowska, A.; Kasprzyk, M.; Ledwójcik, G. Application of Halloysite Nanotubes in Cancer Therapy—A Review. Materials 2021, 14, 2943. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Poma, P.; Colletti, C.G.; Barattucci, A.; Bonaccorsi, P.M.; Lazzara, G.; Nicotra, G.; Parisi, F.; Salerno, T.M.G.; Spinella, C.; et al. Chemical and Biological Evaluation of Cross-Linked Halloysite-Curcumin Derivatives. Appl. Clay Sci. 2020, 184, 105400. [Google Scholar] [CrossRef]
- Stavitskaya, A.; Batasheva, S.; Vinokurov, V.; Fakhrullina, G.; Sangarov, V.; Lvov, Y.; Fakhrullin, R. Antimicrobial Applications of Clay Nanotube-Based Composites. Nanomaterials 2019, 9, 708. [Google Scholar] [CrossRef] [PubMed]
- Stavitskaya, A.; Fakhrullina, G.; Nigamatzyanova, L.; Sitmukhanova, E.; Khusnetdenova, E.; Fakhrullin, R.; Vinokurov, V. Biodistribution of Quantum Dots-Labelled Halloysite Nanotubes: A Caenorhabditis Elegans in Vivo Study. Materials 2021, 14, 5469. [Google Scholar] [CrossRef]
- Stavitskaya, A.V.; Novikov, A.A.; Kotelev, M.S.; Kopitsyn, D.S.; Rozhina, E.V.; Ishmukhametov, I.R.; Fakhrullin, R.F.; Ivanov, E.V.; Lvov, Y.M.; Vinokurov, V.A. Fluorescence and Cytotoxicity of Cadmium Sulfide Quantum Dots Stabilized on Clay Nanotubes. Nanomaterials 2018, 8, 391. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, Y.; Zhou, Y.; Tan, C.; Liu, M. Gold@Halloysite Nanotubes-Chitin Composite Hydrogel with Antibacterial and Hemostatic Activity for Wound Healing. Bioact. Mater. 2023, 20, 355–367. [Google Scholar] [CrossRef]
- Kornilova, A.; Kuralbayeva, G.; Stavitskaya, A.; Gorbachevskii, M.; Karpukhina, O.; Lysenko, I.; Pryadun, V.; Novikov, A.; Vasiliev, A.; Timoshenko, V. Gold Nanoparticles Immobilized on Halloysite Nanotubes for Spatially-Temporally Localized Photohyperthermia. Appl. Surf. Sci. 2021, 566, 150671. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, X.; Wu, Y.P.; Wu, F.; Li, Y.F.; He, R.R.; Liu, M. Rod in Tube: A Novel Nanoplatform for Highly Effective Chemo-Photothermal Combination Therapy toward Breast Cancer. ACS Appl. Mater. Interfaces 2019, 11, 3690–3703. [Google Scholar] [CrossRef]
- Zieba, M.; Hueso, J.L.; Arruebo, M.; Martínez, G.; Santamaría, J. Gold-Coated Halloysite Nanotubes as Tunable Plasmonic Platforms. New J. Chem. 2014, 38, 2037–2042. [Google Scholar] [CrossRef]
- Tan, C.; Zheng, J.; Feng, Y.; Liu, M. Cell Membrane-Coated Halloysite Nanotubes for Target-Specific Nanocarrier for Cancer Phototherapy. Molecules 2021, 26, 4483. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Fiore, B.; la Parola, V.; Lazzara, G.; Guernelli, S.; Zaccheroni, N.; Riela, S. Gold Nanoparticles Stabilized by Modified Halloysite Nanotubes for Catalytic Applications. Appl. Organomet. Chem. 2019, 33, e4665. [Google Scholar] [CrossRef]
- Voronin, D.; Demina, P.; Abramova, A.; Cherednichenko, K.; Vinokurov, V. Freezing-Induced Loading of Au Nanoparticles into Halloysite Nanotubes. Mater. Lett. 2021, 291, 129506. [Google Scholar] [CrossRef]
- Dey, P.; Baumann, V.; Rodríguez-Fernández, J. Gold Nanorod Assemblies: The Roles of Hot-Spot Positioning and Anisotropy in Plasmon Coupling and SERS. Nanomaterials 2020, 10, 942. [Google Scholar] [CrossRef] [PubMed]
- Prinz Setter, O.; Movsowitz, A.; Goldberg, S.; Segal, E. Antibody-Functionalized Halloysite Nanotubes for Targeting Bacterial Cells. ACS Appl. Bio. Mater. 2021, 4, 4094–4104. [Google Scholar] [CrossRef]
- German, S.; Novoselova, M.; Bratashov, D.; Demina, P.; Atkin, V.; Voronin, D.; Khlebtsov, B.; Parakhonskiy, B.; Sukhorukov, G.; Gorin, D.A. High-Efficiency Freezing-Induced Loading of Inorganic Nanoparticles and Proteins into Micron-and Submicron-Sized Porous Particles OPEN. Sci. Rep. 2018, 8, 17763. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.R.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of Halloysite Clay Nanotubes by Grafting with γ-Aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Abdullayev, E.; Joshi, A.; Wei, W.; Zhao, Y.; Lvov, Y. Enlargement of Halloysite Clay Nanotube Lumen by Selective Etching of Aluminum Oxide. ACS Nano 2012, 6, 7216–7226. [Google Scholar] [CrossRef]
- Sun, P.; Liu, G.; Lv, D.; Dong, X.; Wu, J.; Wang, D. Effective Activation of Halloysite Nanotubes by Piranha Solution for Amine Modification via Silane Coupling Chemistry. RSC Adv. 2015, 5, 52916–52925. [Google Scholar] [CrossRef]
- Arshavsky-Graham, S.; Ward, S.J.; Massad-Ivanir, N.; Scheper, T.; Weiss, S.M.; Segal, E. Porous Silicon-Based Aptasensors: Toward Cancer Protein Biomarker Detection. ACS Meas. Sci. Au 2021, 1, 82–94. [Google Scholar] [CrossRef]
- Kim, J.; Cho, J.; Seidler, P.M.; Kurland, N.E.; Yadavalli, V.K. Investigations of Chemical Modifications of Amino-Terminated Organic Films on Silicon Substrates and Controlled Protein Immobilization. Langmuir 2010, 26, 2599–2608. [Google Scholar] [CrossRef]
- Boyle, M.D.P.; Reis, K.J. Bacterial Fc Receptors. Nat. Biotechnol. 1987, 5, 697–703. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold Nanoparticles: Optical Properties and Implementations in Cancer Diagnosis and Photothermal Therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Alkilany, A.M.; Khalil, E.A.; Al-Bakri, A.G. Nano-Photothermal Ablation Effect of Hydrophilic and Hydrophobic Functionalized Gold Nanorods on Staphylococcus Aureus and Propionibacterium Acnes. Sci. Rep. 2018, 8, 6881. [Google Scholar] [CrossRef] [PubMed]
- Mahler, G.J.; Esch, M.B.; Glahn, R.P.; Shuler, M.L. Characterization of a Gastrointestinal Tract Microscale Cell Culture Analog Used to Predict Drug Toxicity. Biotechnol. Bioeng. 2009, 104, 193–205. [Google Scholar] [CrossRef]
- Mahler, G.J.; Shuler, M.L.; Glahn, R.P. Characterization of Caco-2 and HT29-MTX Cocultures in an in Vitro Digestion/Cell Culture Model Used to Predict Iron Bioavailability. J. Nutr. Biochem. 2009, 20, 494–502. [Google Scholar] [CrossRef]
- Lai, X.; Agarwal, M.; Lvov, Y.M.; Pachpande, C.; Varahramyan, K.; Witzmann, F.A. Proteomic Profiling of Halloysite Clay Nanotube Exposure in Intestinal Cell Co-Culture. J. Appl. Toxicol. 2013, 33, 1316–1329. [Google Scholar] [CrossRef]
- Di Paola, M.; Quarta, A.; Pisani, P.; Conversano, F.; Sbenaglia, E.A.; Leporatti, S.; Gigli, G.; Casciaro, S. Surface Coating Highly Improves Cytocompatibility of Halloysite Nanotubes: A Metabolic and Ultrastructural Study. IEEE Trans. Nanotechnol. 2016, 15, 770–774. [Google Scholar] [CrossRef]
- Choi, H.J.; Stazak, T.J.; Montemagno, C.D. Surface-Dependent Cytotoxicity on Bacteria as a Model for Environmental Stress of Halloysite Nanotubes. J. Nanoparticle Res. 2013, 15, 2008. [Google Scholar] [CrossRef]
- Li, W.; Geng, X.; Liu, D.; Li, Z. Near-Infrared Light-Enhanced Protease-Conjugated Gold Nanorods As A Photothermal Antimicrobial Agent For Elimination Of Exotoxin And Biofilms. Int. J. Nanomed. 2019, 14, 8047–8058. [Google Scholar] [CrossRef]
- Tian, Q.; Jiang, F.; Zou, R.; Liu, Q.; Chen, Z.; Zhu, M.; Yang, S.; Wang, J.; Wang, J.; Hu, J. Hydrophilic Cu9S5 Nanocrystals: A Photothermal Agent with a 25.7% Heat Conversion Efficiency for Photothermal Ablation of Cancer Cells in Vivo. ACS Nano 2011, 5, 9761–9771. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.; Fu, F.; Xu, K.; Zou, R.; Wang, Q.; Zhang, B.; Chen, Z.; Hu, J. Facile Synthesis of Biocompatible Cysteine-Coated CuS Nanoparticles with High Photothermal Conversion Efficiency for Cancer Therapy. Dalton Trans. 2014, 43, 11709–11715. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Martínez, J.C.; Martinez-Castanon, G.A.; Martínez-Gutierrez, F.; Zavala-Alonso, N.V.; Patiño-Marín, N.; Niño-Martinez, N.; Zaragoza-Magaña, V.; Cabral-Romero, C. Antibacterial and Antibiofilm Activities of the Photothermal Therapy Using Gold Nanorods against Seven Different Bacterial Strains. J. Nanomater. 2015, 2015, 783671. [Google Scholar] [CrossRef]
- Al-Bakri, A.G.; Mahmoud, N.N.; Estelrich, J.; Busquets, M.A. Photothermal-Induced Antibacterial Activity of Gold Nanorods Loaded into Polymeric Hydrogel against Pseudomonas aeruginosa Biofilm. Molecules 2019, 24, 2661. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Hu, Z.; Saran, A.; Hou, S.; Wen, T.; Liu, W.; Ji, Y.; Jiang, X.; Wu, X. Gold Nanorods Core/AgPt Alloy Nanodots Shell: A Novel Potent Antibacterial Nanostructure. Nano Res. 2013, 6, 822–835. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prinz Setter, O.; Snoyman, I.; Shalash, G.; Segal, E. Gold Nanorod-Incorporated Halloysite Nanotubes Functionalized with Antibody for Superior Antibacterial Photothermal Treatment. Pharmaceutics 2022, 14, 2094. https://doi.org/10.3390/pharmaceutics14102094
Prinz Setter O, Snoyman I, Shalash G, Segal E. Gold Nanorod-Incorporated Halloysite Nanotubes Functionalized with Antibody for Superior Antibacterial Photothermal Treatment. Pharmaceutics. 2022; 14(10):2094. https://doi.org/10.3390/pharmaceutics14102094
Chicago/Turabian StylePrinz Setter, Ofer, Iser Snoyman, Ghazal Shalash, and Ester Segal. 2022. "Gold Nanorod-Incorporated Halloysite Nanotubes Functionalized with Antibody for Superior Antibacterial Photothermal Treatment" Pharmaceutics 14, no. 10: 2094. https://doi.org/10.3390/pharmaceutics14102094