Evaluation of Newly Designed and Traditional Punches in Manufacturing of Scored ODTs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
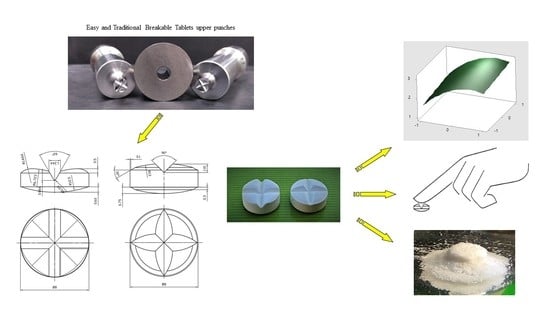
2.2. Manufacturing and Characterization of Tablets
2.3. Breakability Panel Test
2.4. Statistical Analysis
3. Results
3.1. DoE Evaluation of EBT and TBT Punches
| Levels | ||||
|---|---|---|---|---|
| n° | Factor | −1 | 0 | +1 |
| 1 | Compaction force, Fa (kN) | 4.8 | 6.0 | 7.2 |
| 2 | Tablet weight, TW (mg) | 160 | 200 | 240 |
| 3 | Press rotation speed, RS (rpm) | 10 | - | 20 |
| 4 | Punch type (PT) | EBT | - | TBT |
3.2. Panel Test Evaluation of EBT and TBT Punches
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guidance for Industry-Orally Disintegrating Tablets; Office of Pharmaceutical Science in the Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration: Silver Spring, MD, USA, 2008.
- Saurabh, S.; Rajni, B.; Baibhav, J.; Rana, A.C.; Vikas, S. Mouth dissolving tablets: A future compaction. Int. Res. J. Pharm. 2012, 3, 98–109. [Google Scholar]
- Abay, F.B.; Ugurlu, T. Orally Disintegrating Tablets: A Short Review. J. Pharm. Drug Dev. 2015, 3, 1–8. [Google Scholar]
- Badgujar, B.P.; Mundada, A.S. The technologies used for developing orally disintegrating tablets: A review. Acta Pharm. 2011, 61, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Jassem, N.A. Orodispersible Tablets: A Review on Recent Trends in Drug Delivery. Int. J. Drug Deliv. 2022, 12, 432–436. [Google Scholar]
- Tranova, T.; Macho, O.; Loskot, J.; Muzikova, J. Study of rheological and tableting properties of lubricated mixtures of co-processed dry binders for orally disintegrating tablets. Eur. J. Pharm. Sci. 2022, 168, 106035. [Google Scholar] [CrossRef] [PubMed]
- Temer, A.C.; Teixera, M.T.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Silva, I.C.; Taveira, S.F.; Marreto, R.N.; Cunha-Filho, M. Subdivision of tablets containing modified deliver technology: The case of orally disintegrating tablets. J. Pharm. Innov. 2018, 13, 261–269. [Google Scholar] [CrossRef]
- Habib, W.A.; Alanizi, A.S.; Abdelhamid, M.M.; Alanizi, F.K. Accuracy of tablet splitting: Comparison study between hand splitting and tablet cutter. Saudi Pharm. J. 2014, 22, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Van Reuler, A.V.R.; Van Diemen, J.J.K.; Harmsze, A.M.; Fuijkschot, W.W. Subdivision of aspirin tablets? Use your hands: A study on aspirin tablet subdivision using four different methods. J. Pharm. Pract. Res. 2018, 48, 44–48. [Google Scholar] [CrossRef]
- Van Der Steen, K.C.; Frijlink, H.W.; Schipper, C.M.; Barends, D.M. Prediction of the ease of subdivision of scored tablets from their physical parameters. AAPS PharmSciTech 2010, 11, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Rodenhuis, N.; De Smet, P.A.; Barends, D.M. The rationale of scored tablets as dosage form. Eur. J. Pharm. Sci. 2004, 21, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Van Santen, E.; Barends, D.M.; Frijlink, H.W. Breaking of scored tablets: A review. Eur. J. Pharm. Biopharm. 2002, 53, 139–145. [Google Scholar] [CrossRef]
- Green, G.; Berg, C.; Polli, J.E.; Barends, D.M. Pharmacopeial Standards for the Subdivision Characteristics of Scored Tablets. Pharm. Forum 2009, 35, 1598–1612. [Google Scholar]
- Rodenhuis, N.; De Smet, P.A.; Barends, D.M. Patient experiences with the performance of tablet score lines needed for dosing. Pharm. World Sci. 2003, 25, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Palugan, L.; Cerea, M.; Vecchio, C.; Maroni, A.; Foppoli, A.; Moutaharrik, S.; Melocchi, A.; Zema, L.; Gazzaniga, A. Newly designed punch for scored tablets: Evaluation by an expert system based on quality by design. J. Drug. Deliv. Sci. Technol. 2021, 65, 102729. [Google Scholar] [CrossRef]
- Markovic, M.; Zur, M.; Ragatsky, I.; Cvijić, S.; Dahan, A. BCS Class IV oral drugs and absorption windows: Regional-dependent intestinal permeability of furosemide. Pharmaceutics 2020, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Gulsun, T.; Ozturk, N.; Kaynak, M.S.; Vural, I.; Sahin, S. Preparation and evaluation of furosemide containing orally disintegrating tablets by direct compression. Pharmazie 2017, 72, 389–394. [Google Scholar] [PubMed]
- Armstrong, N.A.; James, K.C. Pharmaceutical Experimental Design and Interpretation, 1st ed.; Taylor & Francis: London, UK, 1996; pp. 131–167. [Google Scholar]
- Hooper, P.; Lasher, J.; Alexander, K.S.; Baki, G. A new modified wetting test and an alternative disintegratione test for orally disintegrating tablets. J. Pharm. Biomed. Anal. 2016, 120, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Guidance for Industry-Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation; Office of Pharmaceutical Science in the Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration: Silver Spring, MD, USA, 2013.



| Component | Amount (mg) | ||
|---|---|---|---|
| Furosemide | 25.0 | 25.0 | 25.0 |
| Prosolv® ODT G2 | 130.6 | 169.5 | 208.4 |
| Colloidal silica | 2.0 | 2.5 | 3.0 |
| Magnesium stearate | 2.4 | 3.0 | 3.6 |
| Total mass | 160.0 | 200.0 | 240.0 |
| Trial | Coded Levels | |||
|---|---|---|---|---|
| Compaction Force (Fa) | Tablet Weight (TW) | Press Rotation Speed (RS) | Punch Type (PT) | |
| 1 | −1 | −1 | −1 | −1 |
| 2 | +1 | −1 | −1 | −1 |
| 3 | −1 | +1 | −1 | −1 |
| 4 | +1 | +1 | −1 | −1 |
| 5 | −1 | −1 | +1 | −1 |
| 6 | +1 | −1 | +1 | −1 |
| 7 | −1 | +1 | +1 | −1 |
| 8 | +1 | +1 | +1 | −1 |
| 9 | −1 | −1 | −1 | +1 |
| 10 | +1 | −1 | −1 | +1 |
| 11 | −1 | +1 | −1 | +1 |
| 12 | +1 | +1 | −1 | +1 |
| 13 | −1 | −1 | +1 | +1 |
| 14 | +1 | −1 | +1 | +1 |
| 15 | −1 | +1 | +1 | +1 |
| 16 | +1 | +1 | +1 | +1 |
| 17 | −1 | 0 | −1 | +1 |
| 18 | +1 | 0 | +1 | −1 |
| 19 | 0 | −1 | +1 | −1 |
| 20 | 0 | +1 | −1 | +1 |
| 21 | 0 | 0 | −1 | +1 |
| 22 | 0 | 0 | +1 | −1 |
| 23 | 0 | 0 | +1 | +1 |
| 24 | 0 | 0 | −1 | −1 |
| Batch # | CVT | Fcd• (N) | Fca• (N) | Fri• (%) | WT• (s) | PWU• (%) | DT• (min) | ML• (%) | CVPct |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.09 | 48 ± 5 | 37 ± 5 | 0.485 | 40 ± 4 | 30.1 ± 4.8 | 1.40 ± 0.04 | 0.36 ± 0.29 | 8.32 |
| 2 | 1.30 | 65 ± 7 | 46 ± 4 | 0.311 | 80 ± 25 | 31.3 ± 15.5 | 3.03 ± 0.40 | 0.38 ± 0.28 | 7.85 |
| 3 | 1.17 | 67 ± 8 | 68 ± 10 | 0.630 | 53 ± 8 | 35.9 ± 16.8 | 1.96 ± 0.35 | 0.78 ± 0.42 | 9.60 |
| 4 | 1.59 | 138 ± 9 | 160 ± 17 | 0.362 | 150 ± 46 | 21.9 ± 9.9 | 5.13 ± 0.07 | 0.19 ± 0.11 | 7.65 |
| 5 | 1.30 | 38 ± 7 | 21 ± 4 | 1.049 | 21 ± 1 | 37.7 ± 14.0 | 0.18 ± 0.25 | 1.01 ± 0.49 | 14.74 |
| 6 | 1.86 | 64 ± 7 | 38 ± 5 | 0.065 | 54 ± 28 | 24.9 ± 3.7 | 3.16 ± 0.15 | 0.29 ± 0.29 | 12.69 |
| 7 | 1.62 | 73 ± 6 | 80 ± 11 | 0.666 | 26 ± 15 | 27.2 ± 17.1 | 2.21 ± 0.24 | 0.88 ± 0.56 | 9.98 |
| 8 | 1.10 | 84 ± 20 | 108 ± 13 | 0.343 | 126 ± 3 | 32.9 ± 10.2 | 3.78 ± 0.35 | 0.29 ± 0.12 | 11.05 |
| 9 | 0.96 | 36 ± 8 | 37 ± 7 | 0.368 | 41 ± 12 | 46.0 ± 16.5 | 2.15 ± 0.13 | 2.33 ± 2.82 | 17.08 |
| 10 | 0.89 | 54 ± 9 | 61 ± 9 | 0.187 | 67 ± 18 | 27.5 ± 3.1 | 3.06 ± 0.05 | 0.57 ± 0.35 | 17.85 |
| 11 | 1.35 | 49 ± 13 | 69 ± 12 | 0.341 | 37 ± 28 | 40.3 ± 5.8 | 2.39 ± 0.14 | 1.44 ± 1.09 | 18.84 |
| 12 | 1.36 | 124 ± 13 | 155 ± 17 | 0.041 | 135 ± 23 | 14.6 ± 1.1 | 5.43 ± 0.04 | 0.42 ± 0.33 | 6.35 |
| 13 | 1.29 | 42 ± 8 | 36 ± 5 | 0.551 | 25 ± 9 | 45.6 ± 20.8 | 1.67 ± 0.42 | 3.90 ± 5.53 | 23.40 |
| 14 | 1.06 | 52 ± 7 | 56 ± 4 | 0.285 | 40 ± 10 | 17.9 ± 1.7 | 2.45 ± 0.07 | 0.75 ± 0.36 | 12.82 |
| 15 | 1.81 | 63 ± 11 | 71 ± 16 | 0.395 | 43 ± 10 | 19.8 ± 10.4 | 2.70 ± 0.34 | 2.62 ± 3.07 | 28.07 |
| 16 | 1.35 | 107 ± 18 | 139 ± 21 | 0.247 | 53 ± 5 | 13.8 ± 6.4 | 5.36 ± 0.18 | 0.52 ± 0.32 | 9.56 |
| 17 | 1.97 | 59 ± 11 | 73 ± 8 | 0.253 | 29 ± 10 | 17.8 ± 6.1 | 2.17 ± 0.54 | 1.09 ± 0.60 | 15.78 |
| 18 | 1.28 | 94 ± 16 | 87 ± 8 | 0.317 | 69 ± 17 | 39.6 ± 20.9 | 2.90 ± 0.52 | 0.10 ± 0.41 | 10.99 |
| 19 | 1.91 | 53 ± 13 | 41 ± 10 | 0.351 | 41 ± 6 | 29.8 ± 18.1 | 1.42 ± 0.04 | 0.57 ± 0.43 | 16.60 |
| 20 | 0.82 | 121 ± 9 | 150 ± 10 | 0.164 | 76 ± 18 | 18.5 ± 13.9 | 5.51 ± 0.47 | 0.50 ± 0.51 | 11.84 |
| 21 | 0.80 | 67 ± 7 | 72 ± 9 | 0.304 | 53 ± 12 | 22.9 ± 2.8 | 3.04 ± 0.50 | 0.79 ± 0.41 | 14.66 |
| 22 | 2.10 | 78 ± 8 | 76 ± 9 | 0.376 | 64 ± 4 | 27.5 ± 7.3 | 3.09 ± 0.44 | 0.71 ± 0.87 | 11.89 |
| 23 | 1.56 | 99 ± 19 | 99 ± 11 | 0.049 | 62 ± 37 | 22.4 ± 4.2 | 3.32 ± 0.24 | 1.11 ± 0.64 | 15.13 |
| 24 | 0.78 | 97 ± 13 | 89 ± 8 | 0.220 | 42 ± 15 | 35.0 ± 4.0 | 3.61 ± 0.38 | 0.41 ± 0.22 | 8.79 |
| Coefficient | CVT | Fcd (N) | Fca (N) | Fri (%) | WT (s) | PWU (%) | DT (min) | MLct (%) | CVPct |
|---|---|---|---|---|---|---|---|---|---|
| b0 | 1.347 * | 87.2 * | 87.8 * | 0.244 * | 60 * | 28.33 * | 3.33 * | 0.697 * | 13.31 * |
| b1 | 17.2 * | 21.0 * | −0.158 * | 26 * | −4.54 * | 1.03 * | −0.586 * | −2.63 * | |
| b2 | 20.6 * | 33.7 * | 16 * | −3.34 | 0.83 * | −0.159 | −1.11 | ||
| b3 | 0.173 * | −3.3 | −5.0 | 0.041 | −9 * | −0.25 * | 0.252 * | 1.93 * | |
| b4 | −1.9 | 5.0 | −0.089 * | 5 | −2.88 | 0.28 * | 0.426 * | 2.75 * | |
| b11 | −16.9 * | −13.3 * | 0.151 * | −0.49 | |||||
| b22 | 0.328 | ||||||||
| b12 | 8.1 * | 12.7 * | 12 * | 0.26 | −1.22 | ||||
| b13 | −0.050 | −8 | −0.215 * | −1.12 | |||||
| b14 | −0.054 | 6 | −3.26 | −0.370 * | −2.21 * | ||||
| b24 | −2.82 | −0.186 * | |||||||
| b34 | 4.1 | 0.142 | |||||||
| R2adj | 0.174 | 0.793 | 0.896 | 0.654 | 0.780 | 0.415 | 0.839 | 0.863 | 0.734 |
| R2pred | 0.060 | 0.686 | 0.848 | 0.480 | 0.637 | 0.179 | 0.767 | 0.731 | 0.480 |
| Batch # | Punch | Ds• Arbitrary Units | TB4 % | TB0 % | MLpt• % | CVPpt□ |
|---|---|---|---|---|---|---|
| 1 | EBT | 1.28 ± 1.72 | 96.7 | 0.0 | 1.15 ± 0.47 | 11.10 (58) |
| 24 | EBT | 3.68 ± 2.79 | 83.3 | 8.3 | 0.76 ± 0.31 | 7.14 (50) |
| 9 | TBT | 5.05 ± 2.79 | 71.7 | 10.0 | 0.93 ± 0.42 | 11.74 (43) |
| 21 | TBT | 9.47 ± 1.28 | 8.3 | 81.7 | 0.61 ± 0.31 | 4.78 (5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palugan, L.; Moutaharrik, S.; Maroni, A.; Foppoli, A.A.; Melocchi, A.; Vecchio, C.; Gazzaniga, A.; Cerea, M. Evaluation of Newly Designed and Traditional Punches in Manufacturing of Scored ODTs. Pharmaceutics 2022, 14, 2054. https://doi.org/10.3390/pharmaceutics14102054
Palugan L, Moutaharrik S, Maroni A, Foppoli AA, Melocchi A, Vecchio C, Gazzaniga A, Cerea M. Evaluation of Newly Designed and Traditional Punches in Manufacturing of Scored ODTs. Pharmaceutics. 2022; 14(10):2054. https://doi.org/10.3390/pharmaceutics14102054
Chicago/Turabian StylePalugan, Luca, Saliha Moutaharrik, Alessandra Maroni, Anastasia Anna Foppoli, Alice Melocchi, Carlo Vecchio, Andrea Gazzaniga, and Matteo Cerea. 2022. "Evaluation of Newly Designed and Traditional Punches in Manufacturing of Scored ODTs" Pharmaceutics 14, no. 10: 2054. https://doi.org/10.3390/pharmaceutics14102054