Crystal Structure, Solubility, and Pharmacokinetic Study on a Hesperetin Cocrystal with Piperine as Coformer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of HES–PIP Cocrystal
2.2.2. Single Crystal X-ray Diffraction (SCXRD)
2.2.3. Differential Scanning Calorimetry (DSC) and Thermogravimetric (TG)
2.2.4. High Performance Liquid Chromatography (HPLC)
2.2.5. Powder X-Ray Diffraction (PXRD)
2.2.6. Fourier Transform Infrared (FT-IR)
2.2.7. Solubility Experiments
2.2.8. Bioavailability
3. Results and Discussion
3.1. Cocrystal Screening by DSC
3.2. Crystal Structure Analysis
3.3. Powder PXRD Analysis
3.4. FTIR Analysis
3.5. Analysis of the Solubility Analysis
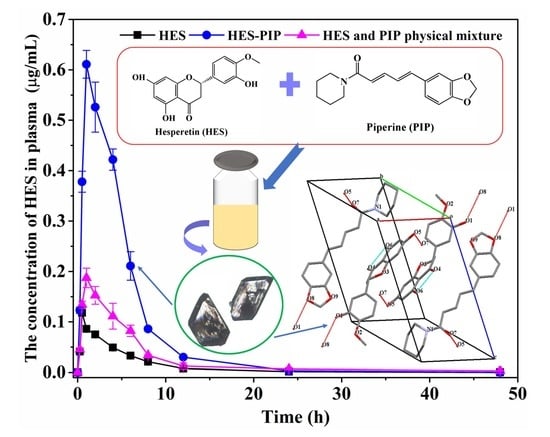
3.6. Bioavailability Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tiekink, E.; Vittal, J.J. Frontiers in Crystal Engineering; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Tawashi, R. Gastrointestinal absorption of two polymorphic forms of aspirin. J. Pharm. Pharmacol. 2011, 21, 701–702. [Google Scholar] [CrossRef]
- Thakuria, R.; Delori, A.; Jones, W.; Lipert, M.P.; Roy, L.; Rodríguez-Hornedo, N. Pharmaceutical cocrystals and poorly soluble drugs. Int. J. Pharm. 2013, 453, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Box, K.J.; Comer, J.; Taylor, R.; Karki, S.; Ruiz, R.; Price, R.; Fotaki, N. Small-Scale Assays for Studying Dissolution of Pharmaceutical Cocrystals for Oral Administration. AAPS PharmSciTech 2015, 17, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Nanda, A. Pharmaceutical Cocrystals: An Overview. Indian J. Pharm. Sci. 2017, 79, 858–871. [Google Scholar] [CrossRef]
- Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical Cocrystals: New Solid Phase Modification Approaches for the Formulation of APIs. Pharmaceutics 2018, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Sathisaran, I.; Dalvi, S.V. Engineering Cocrystals of Poorly Water-Soluble Drugs to Enhance Dissolution in Aqueous Medium. Pharmaceutics 2018, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Roy, P.; Ghosh, A. Progress on cocrystallization of poorly soluble NME’s in the last decade. Crystengcomm 2020, 22, 6958–6974. [Google Scholar] [CrossRef]
- Kumari, N.; Ghosh, A. Cocrystallization: Cutting Edge Tool for Physicochemical Modulation of Active Pharmaceutical Ingredients. Curr. Pharm. Des. 2020, 26, 4858–4882. [Google Scholar] [CrossRef]
- Sible, A.M.; Nawarskas, J.J.; Alajajian, D.; Anderson, J.R. Sacubitril/Valsartan A Novel Cardiovascular Combination Agent. Cardiol. Rev. 2016, 24, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kaduk, J.A.; Gindhart, A.M.; Blanton, T.N. Powder X-ray diffraction of escitalopram oxalate oxalic acid hydrate, (C20H21FN2O)2(C2O4)(H2C2O4)(H2O)0.16. Powder Diffr. 2021, 36, 68–69. [Google Scholar] [CrossRef]
- Videla, S.; Lahjou, M.; Vaqué, A.; Sust, M.; Encabo, M.; Soler, L.; Sans, A.; Sicard, E.; Gascón, N.; Encina, G.; et al. Single-dose pharmacokinetics of co-crystal of tramadol-celecoxib: Results of a four-way randomized open-label phase I clinical trial in healthy subjects. Br. J. Clin. Pharmacol. 2017, 83, 2718–2728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gascon, N.; Almansa, C.; Merlos, M.; Vela, J.M.; Encina, G.; Morte, A.; Smith, K.; Plata-Salamán, C. Co-crystal of tramadol-celecoxib: Preclinical and clinical evaluation of a novel analgesic. Expert Opin. Investig. Drugs 2019, 28, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zou, D.; Chen, X.; Wu, H.; Xu, D. Hesperetin inhibits foam cell formation and promotes cholesterol efflux in THP-1-derived macrophages by activating LXR alpha signal in an AMPK-dependent manner. J. Physiol. Biochem. 2021, 77, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Rehman, T.; Ismael, M.A.; AlAjmi, M.F.; Alruwaished, G.I.; Alokail, M.S.; Khan, M.R. Bioflavonoid (Hesperidin) Restrains Protein Oxidation and Advanced Glycation End Product Formation by Targeting AGEs and Glycolytic Enzymes. Cell Biochem. Biophys. 2021, 79, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, J.; Liu, M.; Zhang, M.; Xue, Y.; Zhang, Y.; Han, X.; Jing, X.; Chu, L. Hesperetin modulates the Sirt1/Nrf2 signaling pathway in counteracting myocardial ischemia through suppression of oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 2021, 139, 111552. [Google Scholar] [CrossRef]
- Teng, J.; Li, J.; Zhao, Y.; Wang, M. Hesperetin, A dietary flavonoid, inhibits AGEs-induced oxidative stress and inflammation in RAW264.7 cells. J. Funct. Foods 2021, 81, 104480. [Google Scholar] [CrossRef]
- Wang, S.-W.; Sheng, H.; Bai, Y.; Weng, Y.; Fan, X.; Zheng, F.; Fu, J.; Zhang, F. Inhibition of histone acetyltransferase by naringenin and hesperetin suppresses Txnip expression and protects pancreatic beta cells in diabetic mice. Phytomedicine 2021, 88, 153454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Y.; Zhang, M.; Chen, Y.; Liu, Y. Hesperetin ameliorates diabetes-associated anxiety and depression-like behaviors in rats via activating Nrf2/ARE pathway. Metab. Brain Dis. 2021, 36, 1969–1983. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Chen, Z.; Qi, X.; Wu, X.; Di, G.; Fan, J.; Guo, C. Improved bioavailability and anticancer efficacy of Hesperetin on breast cancer via a self-assembled rebau-dioside A nanomicelles system. Toxicol. Appl. Pharmacol. 2021, 419, 115511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Gu, W.; Kui, F.; Gao, F.; Niu, Y.; Li, W.; Zhang, Y.; Guo, L.; Wang, J.; Guo, Z.; et al. The mechanism and candidate compounds of aged citrus peel (chenpi) preventing chronic obstructive pulmonary disease and its progression to lung cancer. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- Babylon, L.; Grewal, R.; Stahr, P.-L.; Eckert, R.; Keck, C.; Eckert, G. Hesperetin Nanocrystals Improve Mitochondrial Function in a Cell Model of Early Alzheimer Disease. Antioxidants 2021, 10, 1003. [Google Scholar] [CrossRef]
- Wang, J.; Dai, X.; Dai, X.; Lu, T.; Chen, J. Temozolomide-Hesperetin Drug-Drug Cocrystal with Optimized Performance in Stability, Dissolution, and Tabletability. Cryst. Growth Des. 2021, 21, 838–846. [Google Scholar] [CrossRef]
- Chadha, K.; Karan, M.; Bhalla, Y.; Chadha, R.; Khullar, S.; Mandal, S.; Vasisht, K. Cocrystals of Hesperetin: Structural, Pharmacokinetic, and Pharmacodynamic Evaluation. Cryst. Growth Des. 2017, 17, 2386–2405. [Google Scholar] [CrossRef]
- Emami, S.; Siahi-Shadbad, M.; Adibkia, K.; Barzegar-Jalali, M. Recent advances in improving oral drug bioavailability by cocrystals. BioImpacts 2018, 8, 305–320. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, D.; Kaur, C.D. Multicomponent Pharmaceutical Cocrystals: A Novel Approach for Combination Therapy. Mini-Rev. Med. Chem. 2018, 18, 1160–1167. [Google Scholar] [CrossRef]
- Erxleben, A. Cocrystal Applications in Drug Delivery. Pharmaceutics 2020, 12, 834. [Google Scholar] [CrossRef]
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical cocrystals: A review of preparations, physicochemical properties and applications. Acta Pharm. Sin. B 2021, 11, 2537–2564. [Google Scholar] [CrossRef]
- Wong, S.N.; Chen, Y.C.S.; Xuan, B.; Sun, C.C.; Chow, S.F. Cocrystal engineering of pharmaceutical solids: Therapeutic potential and challenges. CrystEngComm 2021, 23, 7005–7038. [Google Scholar] [CrossRef]
- Peterson, B.; Weyers, M.; Steenekamp, J.H.; Steyn, J.D.; Gouws, C.; Hamman, J.H. Drug Bioavailability Enhancing Agents of Natural Origin (Bioenhancers) that Modulate Drug Membrane Permeation and Pre-Systemic Metabolism. Pharmaceutics 2019, 11, 33. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, J.T.; Tian, D.; Tanna, R.S.; Hadi, D.L.; Bansal, S.; Calamia, J.C.; Arian, C.M.; Shireman, L.M.; Molnár, B.; Horváth, M.; et al. Assessing Transporter-Mediated Natural Product-Drug Interactions Via In vitro—In Vivo Extrapolation: Clinical Evaluation with a Probe Cocktail. Clin. Pharmacol. Ther. 2020, 109, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Ashour, E.A.; Majumdar, S.; Alsheteli, A.; Alshehri, S.; Alsulays, B.; Feng, X.; Gryczke, A.; Kolter, K.; Langley, N.; Repka, M.A. Hot melt extrusion as an approach to improve solubility, permeability and oral absorption of a psychoactive natural product, piperine. J. Pharm. Pharmacol. 2016, 68, 989–998. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Kim, H.Y.; Back, S.Y.; Han, H.-K. Piperine-mediated drug interactions and formulation strategy for piperine: Recent advances and future perspectives. Expert Opin. Drug Metab. Toxicol. 2017, 14, 43–57. [Google Scholar] [CrossRef]
- Slika, L.; Moubarak, A.; Borjac, J.; Baydoun, E.; Patra, D. Preparation of curcumin-poly (allyl amine) hydrochloride based nanocapsules: Piperine in nanocapsules accelerates encapsulation and release of curcumin and effectiveness against colon cancer cells. Mater. Sci. Eng. C 2019, 109, 110550. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J.; Nihal, M.; Siddiqui, I.A.; Scarlett, C.O.; Bailey, H.H.; Mukhtar, H.; Ahmad, N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 2011, 55, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Zhang, Q.; Wang, J.-R.; Mei, X. Structure, Physicochemical properties and pharmacokinetics of resveratrol and piperine cocrystals. CrystEngComm 2017, 19, 6154–6163. [Google Scholar] [CrossRef]
- Yu, D.; Kan, Z.; Shan, F.; Zang, J.; Zhou, J. Triple Strategies to Improve Oral Bioavailability by Fabricating Coamorphous Forms of Ursolic Acid with Piperine: Enhancing Water-Solubility, Permeability, and Inhibiting Cytochrome P450 Isozymes. Mol. Pharm. 2020, 17, 4443–4462. [Google Scholar] [CrossRef] [PubMed]
- Zaini, E.; Afriyani, A.; Fitriani, L.; Ismed, F.; Horikawa, A.; Uekusa, H. Improved Solubility and Dissolution Rates in Novel Multicomponent Crystals of Piperine with Succinic Acid. Sci. Pharm. 2020, 88, 21. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, T.; Freire-De-Lima, L.; Previato, J.O.; Previato, L.M.; Heise, N.; de Lima, M.E.F. Toxic effects of natural piperine and its derivatives on epimastigotes and amastigotes of Trypanosoma cruzi. Bioorg. Med. Chem. Lett. 2004, 14, 3555–3558. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar]
- Guzei, I.A. An idealized molecular geometry library for refinement of poorly behaved molecular fragments with constraints. J. Appl. Crystallogr. 2014, 47, 806–809. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D-Struct. Biol. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Gong, S.; Ding, J.; Yu, M.; Ahmad, E.; Feng, Y.; Gan, Y. Supersaturated polymeric micelles for oral silybin delivery: The role of the Soluplus–PVPVA complex. Acta Pharm. Sin. B 2018, 9, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-T.; Chen, J.-A.; Hsu, C.; Su, N.-W. Microbial Phosphorylation Product of Hesperetin by Bacillus subtilis BCRC 80517 Improves Oral Bioavailability in Rats. J. Agric. Food Chem. 2021, 69, 10184–10193. [Google Scholar] [CrossRef]
- Vasilev, N.A.; Surov, A.O.; Voronin, A.P.; Drozd, K.V.; Perlovich, G.L. Novel cocrystals of itraconazole: Insights from phase diagrams, formation thermodynamics and solubility. Int. J. Pharm. 2021, 599, 120441. [Google Scholar] [CrossRef]
- Yamashita, H.; Hirakura, Y.; Yuda, M.; Teramura, T.; Terada, K. Detection of Cocrystal Formation Based on Binary Phase Diagrams Using Thermal Analysis. Pharm. Res. 2012, 30, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Ervasti, T.; Aaltonen, J.; Ketolainen, J. Theophylline–nicotinamide cocrystal formation in physical mixture during storage. Int. J. Pharm. 2015, 486, 121–130. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Lin, H.-L.; Wang, S.-L.; Lin, S.-Y. Solid-state thermal behavior and stability studies of theophylline–citric acid cocrystals prepared by neat cogrinding or thermal treatment. J. Solid State Chem. 2012, 192, 238–245. [Google Scholar] [CrossRef]
- Liu, M.; Hong, C.; Yao, Y.; Shen, H.; Ji, G.; Li, G.; Xie, Y. Development of a pharmaceutical cocrystal with solution crystallization technology: Preparation, characterization, and evaluation of myricetin-proline cocrystals. Eur. J. Pharm. Biopharm. 2016, 107, 151–159. [Google Scholar] [CrossRef]
- Fujii, S.; Yamagata, Y.; Jin, G.-Z.; Tomita, K.-I. Novel Molecular Conformation of (R,S)-Hesperetin in Anhydrous Crystal. Chem. Pharm. Bull. 1994, 42, 1143–1145. [Google Scholar] [CrossRef] [Green Version]
- Unsalan, O.; Erdogdu, Y.; Gulluoglu, M.T. FT-Raman and FT-IR spectral and quantum chemical studies on some flavonoid derivatives: Baicalein and Naringenin. J. Raman Spectrosc. 2009, 40, 562–570. [Google Scholar] [CrossRef]
- Bavishi, D.D.; Borkhataria, C.H. Borkhataria, Spring and parachute: How cocrystals enhance solubility. Prog. Cryst. Growth Charact. Mater. 2016, 62, 1–8. [Google Scholar] [CrossRef]
- Chu, K.A.; Yalkowsky, S.H. An interesting relationship between drug absorption and melting point. Int. J. Pharm. 2009, 373, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.V.; Godbole, M.M.; Misra, K. A plausible explanation for enhanced bioavailability of P-gp substrates in presence of piperine: Simulation for next generation of P-gp inhibitors. J. Mol. Model. 2012, 19, 227–238. [Google Scholar] [CrossRef] [PubMed]







| Compound | HSP–PIP |
|---|---|
| Chemical formula | C33H33NO9 |
| Formula weight | 587.60 |
| Crystal size (mm) | 0.14 × 0.12 × 0.10 |
| Temperature (K) | 296 (2) |
| Radiation (Å) | 0.71073 |
| Crystal system | Triclinic |
| Space group | P −1 |
| a (Å) | 10.531 (2) |
| b (Å) | 11.879 (3) |
| c (Å) | 13.363 (3) |
| α (°) | 105.644 (2) |
| β (°) | 111.934 (2) |
| γ (°) | 100.486 (2) |
| V (Å3) | 1416.4 (5) |
| Z | 2 |
| ρ(calc) (g/cm3) | 1.378 |
| F (000) | 620 |
| absorp.coeff. (mm−1) | 0.101 |
| θ range (deg) | 2.86 to 25.02 |
| reflns collected | (Rint = 0.0143) |
| indep. reflns | 4983 |
| Refns obs. [I > 2σ(I)] | 4331 |
| data/restr/paras | 4983/0/392 |
| GOF | 1.024 |
| R1/wR2 [I > 2σ(I)] | 0.0405/0.1079 |
| R1/wR2 (all data) | 0.0462/0.1071 |
| larg peak and hole (e/Å3) | 0.421/−0.265 |
| Hydrogen Bond | H-A (Å) | D-A (Å) | <D-H-A (Deg) | Symmetry Code |
|---|---|---|---|---|
| O1H1O2 | 2.220 | 2.667 | 114.55 | |
| O1H1O8 | 2.243 | 2.845 | 130.52 | x − 2, y − 1, z − 1 |
| O5H5O7 | 1.865 | 2.679 | 171.75 | −x + 1, −y + 2, −z + 1 |
| O6H6AO4 | 1.870 | 2.600 | 147.63 |
| Parameters | HES | HES–PIP | HES + PIP |
|---|---|---|---|
| Cmax (µg/mL) | 0.12 | 0.61 | 0.19 |
| Tmax (h) | 0.5 | 1 | 1 |
| AUC(0–t) (µg/mL*h) | 0.53 | 3.23 | 1.17 |
| t1/2 (h) | 3.01 | 2.68 | 3.26 |
| MRT(0–t) (h) | 5.86 | 4.47 | 7.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yang, F.; Zhao, X.; Wang, S.; Yang, Q.; Zhang, X. Crystal Structure, Solubility, and Pharmacokinetic Study on a Hesperetin Cocrystal with Piperine as Coformer. Pharmaceutics 2022, 14, 94. https://doi.org/10.3390/pharmaceutics14010094
Liu Y, Yang F, Zhao X, Wang S, Yang Q, Zhang X. Crystal Structure, Solubility, and Pharmacokinetic Study on a Hesperetin Cocrystal with Piperine as Coformer. Pharmaceutics. 2022; 14(1):94. https://doi.org/10.3390/pharmaceutics14010094
Chicago/Turabian StyleLiu, Yanjie, Fan Yang, Xiuhua Zhao, Siying Wang, Qilei Yang, and Xiaoxue Zhang. 2022. "Crystal Structure, Solubility, and Pharmacokinetic Study on a Hesperetin Cocrystal with Piperine as Coformer" Pharmaceutics 14, no. 1: 94. https://doi.org/10.3390/pharmaceutics14010094