Combined Curcumin and Lansoprazole-Loaded Bioactive Solid Self-Nanoemulsifying Drug Delivery Systems (Bio-SSNEDDS)
Abstract
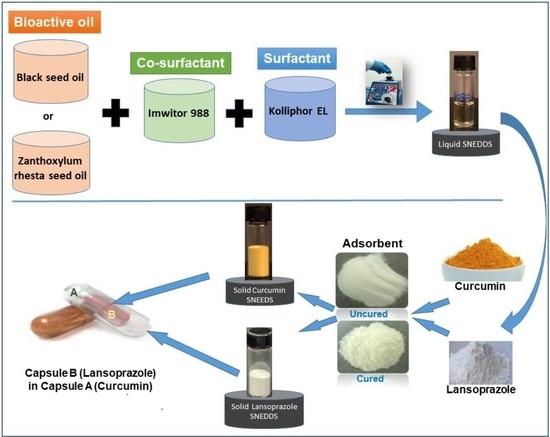
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction of Bioactive Oils
2.2. Chemical and Reagents
2.3. Analysis of Curcumin (CUR) and Lansoprazole (LNS) Using Ultra-High Performance Liquid Chromatography (UHPLC)
2.3.1. UHPLC Chromatographic Conditions
2.3.2. Linearity and Calibration
2.3.3. Accuracy and Precision
2.3.4. Limit of Detection (LOD) and Lower Limit of Quantification (LLOQ)
2.4. Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) Development and Characterization
2.4.1. Preparation and Drug Loading
2.4.2. Formulation Assessment and Characterization
2.5. Solidification of CUR and LNS Loaded Liquid SNEDDS Using Adsorbent Neusilin® US2
2.5.1. Curing Process of the Adsorbent Neusilin® US2
2.5.2. Brunauer–Emmett–Teller (BET) Surface Area of Cured/Uncured Adsorbent
2.5.3. Preparation of Solid SNEDDS Using Uncured/Cured NUS2
2.6. Characterization of Solid SNEDDS
2.6.1. Scanning Electron Microscopy (SEM) Powder SNEDDS
2.6.2. Differential Scanning Calorimetry (DSC)
2.6.3. X-ray Diffraction (XRD)
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Filling of Solid CUR-SNEDDS into Capsules
2.9. In Vitro Dissolution Studies
2.10. Accelerated Stability Study
2.11. Statistical Analysis
3. Results
3.1. Optimization of UHPLC Peak Separations
3.1.1. Linearity and Calibration
3.1.2. Accuracy and Precision
3.1.3. Limit of Detection and Quantification
3.1.4. Method Application and Matrix Effect: Determination of CUR and LNS in Marketed Product of Future-Biotics® and Ultrazole®
3.2. Liquid SNEDDS Development and Performance
3.3. Solidification of Liquid SNEDDS on Neusilin US2
3.3.1. Physical Appearance
3.3.2. Brunauer–Emmett–Teller (BET) Surface Area
3.4. Characterization of Solid SNEDDS
3.4.1. Scanning Electron Microscopy (SEM)
3.4.2. Differential Scanning Calorimetry (DSC)
3.4.3. X-ray Powder Diffraction (XRD)
3.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.5. In Vitro Dissolution of Curcumin and Lansoprazole
3.6. Stability at Accelerated Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pok, L.S.L.; Shabaruddin, F.H.; Dahlui, M.; Sockalingam, S.; Mohamed Said, M.S.; Rosman, A.; Lau, I.S.; Isa, L.M.; Hussein, H.; Ng, C.T.; et al. Clinical and economic implications of upper gastrointestinal adverse events in Asian rheumatological patients on long-term non-steroidal anti-inflammatory drugs. Int. J. Rheum. Dis. 2018, 21, 943–951. [Google Scholar] [CrossRef]
- Albeiruti, R.; Chaudhary, F.; Vallabh, H.; Krupica, T.; Kupec, J. Fungal Peptic Ulcer Disease in an Immunocompetent Patient. Eur. J. Case Rep. Intern. Med. 2020, 7, 001696. [Google Scholar] [CrossRef]
- Azhari, H.; Underwood, F.; King, J.; Coward, S.; Shah, S.; Chan, C.; Ho, G.; Ng, S.; Kaplan, G. The Global Incidence of Peptic Ulcer Disease and Its Complications at the Turn of the 21st Century: A Systematic Review: 1199. Off. J. Am. Coll. Gastroenterol. 2018, 113, S684–S685. [Google Scholar] [CrossRef]
- Katz, P.O.; Gerson, L.B.; Vela, M.F. Guidelines for the Diagnosis and Management of Gastroesophageal Reflux Disease. Off. J. Am. Coll. Gastroenterol. 2013, 108, 308–328. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Sweet, S.; Winchester, C.C.; Dent, J. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 2014, 63, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Baldi, F.; Malfertheiner, P. Lansoprazole fast disintegrating tablet: A new formulation for an established proton pump inhibitor. Digestion 2003, 67, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Boparai, V.; Rajagopalan, J.; Triadafilopoulos, G. Guide to the use of proton pump inhibitors in adult patients. Drugs 2008, 68, 925–947. [Google Scholar] [CrossRef]
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Singla, V.; Pratap Mouli, V.; Garg, S.K.; Rai, T.; Choudhury, B.N.; Verma, P.; Deb, R.; Tiwari, V.; Rohatgi, S.; Dhingra, R. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis—a randomized, placebo-controlled, pilot study. J. Crohn′s Colitis 2014, 8, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.-C.; Zhang, B.; Liao, M.-J.; Zhang, W.-X.; He, W.-Y.; Wang, H.-B.; Yang, C.-X. Curcumin ameliorated diabetic neuropathy partially by inhibition of NADPH oxidase mediating oxidative stress in the spinal cord. Neurosci. Lett. 2014, 560, 81–85. [Google Scholar] [CrossRef]
- Yu, C.-W.; Wei, C.-C.; Liao, V.-C. Curcumin-mediated oxidative stress resistance in Caenorhabditis elegans is modulated by age-1, akt-1, pdk-1, osr-1, unc-43, sek-1, skn-1, sir-2.1, and mev-1. Free Radic. Res. 2014, 48, 371–379. [Google Scholar] [CrossRef]
- Sanmukhani, J.; Satodia, V.; Trivedi, J.; Patel, T.; Tiwari, D.; Panchal, B.; Goel, A.; Tripathi, C.B. Efficacy and safety of curcumin in major depressive disorder: A randomized controlled trial. Phytother. Res. 2014, 28, 579–585. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, J.; Zhang, M.; Yao, W.; Ma, X.; Yu, S.Y. Effects of curcumin on chronic, unpredictable, mild, stress-induced depressive-like behaviour and structural plasticity in the lateral amygdala of rats. Int. J. Neuropsychopharmacol. 2014, 17, 793–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, K.M.; Mishra, A.; Poroikov, V.V.; Goel, R.K. Ameliorative effect of Curcumin on seizure severity, depression like behavior, learning and memory deficit in post-pentylenetetrazole-kindled mice. Eur. J. Pharmacol. 2013, 704, 33–40. [Google Scholar] [CrossRef]
- Lim, T.-G.; Lee, S.-Y.; Huang, Z.; Chen, H.; Jung, S.K.; Bode, A.M.; Lee, K.W.; Dong, Z. Curcumin suppresses proliferation of colon cancer cells by targeting CDK2. Cancer Prev. Res. 2014, 7, 466–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-F.; Zu, J.-N.; Li, J.; Chen, C.; Xi, C.-Y.; Yan, J.-L. Curcumin promotes the spinal cord repair via inhibition of glial scar formation and inflammation. Neurosci. Lett. 2014, 560, 51–56. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Daglia, M.; Moghaddam, A.H.; Habtemariam, S.; Nabavi, S.M. Curcumin and liver disease: From chemistry to medicine. Compr. Rev. Food Sci. Food Saf. 2014, 13, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wu, C.; Lin, Z.; Guo, Y.; Shi, L.; Dong, P.; Lu, Z.; Gao, S.; Liao, Y.; Chen, B. Curcumin up-regulates phosphatase and tensin homologue deleted on chromosome 10 through microRNA-mediated control of DNA methylation–a novel mechanism suppressing liver fibrosis. FEBS J. 2014, 281, 88–103. [Google Scholar] [CrossRef]
- Mahattanadul, S.; Nakamura, T.; Panichayupakaranant, P.; Phdoongsombut, N.; Tungsinmunkong, K.; Bouking, P. Comparative antiulcer effect of bisdemethoxycurcumin and curcumin in a gastric ulcer model system. Phytomedicine 2009, 16, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Alwadei, M.; Kazi, M.; Alanazi, F.K. Novel oral dosage regimen based on self-nanoemulsifying drug delivery systems for codelivery of phytochemicals—Curcumin and thymoquinone. Saudi Pharm. J. 2019, 27, 866–876. [Google Scholar] [CrossRef]
- Mahattanadul, S.; Radenahmad, N.; Phadoongsombut, N.; Chuchom, T.; Panichayupakaranant, P.; Yano, S.; Reanmongkol, W. Effects of curcumin on reflux esophagitis in rats. J. Nat. Med. 2006, 60, 198–205. [Google Scholar] [CrossRef]
- Kositchaiwat, C.; Kositchaiwat, S.; Havanondha, J. Curcuma longa Linn. in the treatment of gastric ulcer comparison to liquid antacid: A controlled clinical trial. J. Med. Assoc. Thail. 1993, 76, 601–605. [Google Scholar]
- Prucksunand, C.; Indrasukhsri, B.; Leethochawalit, M.; Hungspreugs, K. Phase II clinical trial on effect of the long turmeric (Curcuma longa Linn) on healing of peptic ulcer. Southeast Asian J. Trop. Med. Public Health 2001, 32, 208–215. [Google Scholar] [PubMed]
- Mermelstein, J.; Chait Mermelstein, A.; Chait, M.M. Proton pump inhibitor-refractory gastroesophageal reflux disease: Challenges and solutions. Clin. Exp. Gastroenterol. 2018, 11, 119–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazi, M.; Alhajri, A.; Alshehri, S.; Elzayat, E.; Meanazel, O.T.A.; Shakeel, F.; Noman, O.M.; Altamimi, M.A.; Alanazi, F. Enhancing Oral Bioavailability of Apigenin Using a Bioactive Self-Nanoemulsifying Drug Delivery System (Bio-SNEDDS): In Vitro, In Vivo and Stability Evaluations. Pharmaceutics 2020, 12, 749. [Google Scholar] [CrossRef]
- Kazi, M.; Shahba, A.A.; Alrashoud, S.; Alwadei, M.; Sherif, A.Y.; Alanazi, F.K. Bioactive Self-Nanoemulsifying Drug Delivery Systems (Bio-SNEDDS) for Combined Oral Delivery of Curcumin and Piperine. Molecules 2020, 25, 1703. [Google Scholar] [CrossRef] [Green Version]
- Hosny, K.; Asfour, H.; Rizg, W.; Alhakamy, N.A.; Sindi, A.; Alkhalidi, H.; Abualsunun, W.; Bakhaidar, R.; Almehmady, A.M.; Akeel, S.; et al. Formulation, Optimization, and Evaluation of Oregano Oil Nanoemulsions for the Treatment of Infections Due to Oral Microbiota. Int. J. Nanomed. 2021, 16, 5465–5478. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; El-Bassossy, H.M.; El-Halawany, A.M.; Ahmed, T.A.; Mohamed, G.A.; Malebari, A.M.; Hassan, N.A. Self-Nanoemulsifying Drug Delivery System Loaded with Psiadia punctulata Major Metabolites for Hypertensive Emergencies: Effect on Hemodynamics and Cardiac Conductance. Front. Pharmacol. 2021, 12, 681070. [Google Scholar] [CrossRef]
- Rich, G.; Shah, A.; Koloski, N.; Funk, P.; Stracke, B.; Köhler, S.; Holtmann, G. A randomized placebo-controlled trial on the effects of Menthacarin, a proprietary peppermint- and caraway-oil-preparation, on symptoms and quality of life in patients with functional dyspepsia. Neurogastroenterol. Motil. 2017, 29, e13132. [Google Scholar] [CrossRef]
- Mohtashami, R.; Huseini, H.F.; Heydari, M.; Amini, M.; Sadeqhi, Z.; Ghaznavi, H.; Mehrzadi, S. Efficacy and safety of honey based formulation of Nigella sativa seed oil in functional dyspepsia: A double blind randomized controlled clinical trial. J. Ethnopharmacol. 2015, 175, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, M.; Hibbert, D.B. Linearity and the limitations of least squares calibration. J. Chromatogr. A 1997, 762, 73–82. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Shahba, A.; Mohsin, K.; Alanazi, F. Ultra performance liquid chromatography assay for cinnarizine in lipid-based formulations. Asian J. Chem. 2012, 24, 595–600. [Google Scholar]
- Shahba, A.A.; Tashish, A.Y.; Alanazi, F.K.; Kazi, M. Combined Self-Nanoemulsifying and Solid Dispersion Systems Showed Enhanced Cinnarizine Release in Hypochlorhydria/Achlorhydria Dissolution Model. Pharmaceutics 2021, 13, 627. [Google Scholar] [CrossRef] [PubMed]
- Shahba, A.A.; Ahmed, A.R.; Mohsin, K.; Abdel-Rahman, S.I.; Alanazi, F.K. Solidification of cinnarizine self-nanoemulsifying drug delivery systems by fluid bed coating: Optimization of the process and formulation variables. Pharmazie 2017, 72, 143–151. [Google Scholar] [CrossRef]
- Mohsin, K.; Long, M.A.; Pouton, C.W. Design of lipid-based formulations for oral administration of poorly water-soluble drugs: Precipitation of drug after dispersion of formulations in aqueous solution. J. Pharm. Sci. 2009, 98, 3582–3595. [Google Scholar] [CrossRef]
- Gumaste, S.G.; Freire, B.O.S.; Serajuddin, A.T.M. Development of solid SEDDS, VI: Effect of precoating of Neusilin((R)) US2 with PVP on drug release from adsorbed self-emulsifying lipid-based formulations. Eur. J. Pharm. Sci. 2017, 110, 124–133. [Google Scholar] [CrossRef]
- Gumaste, S.G.; Serajuddin, A.T.M. Development of solid SEDDS, VII: Effect of pore size of silica on drug release from adsorbed self-emulsifying lipid-based formulations. Eur. J. Pharm. Sci. 2017, 110, 134–147. [Google Scholar] [CrossRef]
- Patki, M.; Patel, K. Development of a solid supersaturated self-nanoemulsifying preconcentrate (S-superSNEP) of fenofibrate using dimethylacetamide and a novel co-processed excipient. Drug Dev. Ind. Pharm. 2019, 45, 405–414. [Google Scholar] [CrossRef]
- Alhasani, K.F.; Kazi, M.; Ibrahim, M.A.; Shahba, A.A.; Alanazi, F.K. Self-nanoemulsifying ramipril tablets: A novel delivery system for the enhancement of drug dissolution and stability. Int. J. Nanomed. 2019, 14, 5435–5448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahba, A.A.-W.; Mohsin, K.; Alanazi, F.K. Novel self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of cinnarizine: Design, optimization, and in-vitro assessment. AAPS PharmSciTech 2012, 13, 967–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Maghraby, G.M.; Elzayat, E.M.; Alanazi, F.K. Development of modified in situ gelling oral liquid sustained release formulation of dextromethorphan. Drug Dev. Ind. Pharm. 2012, 38, 971–978. [Google Scholar] [CrossRef]
- Shahba, A.A.; Ahmed, A.R.; Alanazi, F.K.; Mohsin, K.; Abdel-Rahman, S.I. Multi-Layer Self-Nanoemulsifying Pellets: An Innovative Drug Delivery System for the Poorly Water-Soluble Drug Cinnarizine. AAPS PharmSciTech 2018, 19, 2087–2102. [Google Scholar] [CrossRef]
- Shahba, A.A.; Alanazi, F.K.; Abdel-Rahman, S.I. Stabilization benefits of single and multi-layer self-nanoemulsifying pellets: A poorly-water soluble model drug with hydrolytic susceptibility. PLoS ONE 2018, 13, e0198469. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry: Bioanalytical Method Validation. 2001. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 6 December 2021).
- Imran, S.M.; Moiz, S.A.; Ahmed, M.M.; Lee, J.; Kim, H. Structural characterization of the silver-polyaniline nanocomposite for electronic devices. In Proceedings of the 2009 IEEE 13th International Multitopic Conference, Islamabad, Pakistan, 14–15 December 2009; pp. 1–4. [Google Scholar]
- Silva, H.D.; Cerqueira, M.A.; Vicente, A.A. Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. J. Food Eng. 2015, 167, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Chen, L.; Zhang, W. Influence of Ionic Surfactants on the Properties of Nanoemulsions Emulsified by Nonionic Surfactants Span 80/Tween 80. J. Dispers. Sci. Technol. 2016, 37, 1511–1517. [Google Scholar] [CrossRef]
- Penjuri, S.C.B.; Ravouru, N.; Damineni, S.; Bns, S.; Poreddy, S.R. Formulation and evaluation of lansoprazole loaded Nanosponges. Turk. J. Pharm. Sci. 2016, 13, 304–310. [Google Scholar] [CrossRef]
- Rosenblatt, K.M.; Bunjes, H.; Seeling, A.; Oelschläger, H. Investigations on the thermal behavior of omeprazole and other sulfoxides. Pharmazie 2005, 60, 503–507. [Google Scholar] [PubMed]
- Sharma, V.; Pathak, K. Effect of hydrogen bond formation/replacement on solubility characteristics, gastric permeation and pharmacokinetics of curcumin by application of powder solution technology. Acta Pharm. Sin. B 2016, 6, 600–613. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Guo, T.; Qi, J.; Zhang, J.; Wu, W. Enhanced dissolution and stability of lansoprazole by cyclodextrin inclusion complexation: Preparation, characterization, and molecular modeling. AAPS PharmSciTech 2012, 13, 1222–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, P.S.; Kumar, V.; Singh, U.P.; Bhat, H.R.; Mazumder, B. Physicochemical characterization and in vitro dissolution studies of solid dispersions of ketoprofen with PVP K30 and d-mannitol. Saudi Pharm. J. 2013, 21, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Chhouk, K.; Diono, W.; Kanda, H.; Goto, M. Micronization for Enhancement of Curcumin Dissolution via Electrospraying Technique. ChemEngineering 2018, 2, 60. [Google Scholar] [CrossRef] [Green Version]
- Kazi, M.; Nasr, F.A.; Noman, O.; Alharbi, A.; Alqahtani, M.S.; Alanazi, F.K. Development, Characterization Optimization, and Assessment of Curcumin-Loaded Bioactive Self-Nanoemulsifying Formulations and Their Inhibitory Effects on Human Breast Cancer MCF-7 Cells. Pharmaceutics 2020, 12, 1107. [Google Scholar] [CrossRef]
- Nguyen, T.N.-G.; Tran, P.H.-L.; Van Vo, T.; Van Tran, T.; Tran, T.T.-D. Dissolution Enhancement of Curcumin by Solid Dispersion with Polyethylene Glycol 6000 and Hydroxypropyl Methylcellulose. In Proceedings of the 5th International Conference on Biomedical Engineering in Vietnam, Ho Chi Minh City, Vietnam, 16–18 June 2014; pp. 298–301. [Google Scholar]
- Gangurde, A.B.; Kundaikar, H.S.; Javeer, S.D.; Jaiswar, D.R.; Degani, M.S.; Amin, P.D. Enhanced solubility and dissolution of curcumin by a hydrophilic polymer solid dispersion and its insilico molecular modeling studies. J. Drug Deliv. Sci. Technol. 2015, 29, 226–237. [Google Scholar] [CrossRef]
- Gumaste, S.G.; Dalrymple, D.M.; Serajuddin, A.T.M. Development of Solid SEDDS, V: Compaction and Drug Release Properties of Tablets Prepared by Adsorbing Lipid-Based Formulations onto Neusilin® US2. Pharm. Res. 2013, 30, 3186–3199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Dong, J.; Chen, J.; Eastoe, J.; Li, X. Design and optimization of a new self-nanoemulsifying drug delivery system. J. Colloid Interface Sci. 2009, 330, 443–448. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Al-Zehouri, J.; El-Subbagh, H.I.; Al-Badr, A.A. Lansoprazole. In Analytical Profiles of Drug Substances and Excipients; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2001; Volume 28, pp. 117–151. [Google Scholar]
- DellaGreca, M.; Iesce, M.R.; Previtera, L.; Rubino, M.; Temussi, F.; Brigante, M. Degradation of lansoprazole and omeprazole in the aquatic environment. Chemosphere 2006, 63, 1087–1093. [Google Scholar] [CrossRef]
- Byrn, S.R.; Xu, W.; Newman, A.W. Chemical reactivity in solid-state pharmaceuticals: Formulation implications. Adv. Drug Deliv. Rev. 2001, 48, 115–136. [Google Scholar] [CrossRef]












| Formulation Code | ZRO (%) | I988 (%) | BSO (%) | KrEL (%) | NUS2 (%) | PVP-K30 (%) |
|---|---|---|---|---|---|---|
| F1 | 35 | 15 | -- | 50 | -- | -- |
| F2 | -- | 15 | 35 | 50 | -- | -- |
| SF1-UC | 17.5 | 7.5 | - | 25 | 50 | -- |
| SF2-UC | -- | 7.5 | 17.5 | 25 | 50 | -- |
| SF1-C | 17.5 | 7.5 | -- | 25 | 45.5 | 4.5 |
| SF2-C | -- | 7.5 | 17.5 | 25 | 45.5 | 4.5 |
| Assay Type | Nominal Drug Concentration (ug/mL) | CUR | LNS | ||
|---|---|---|---|---|---|
| Accuracy (%) | RSD (%) | Accuracy (%) | RSD (%) | ||
| Intra-Day | 0.5 | 89.4 | 7.9 | 99.0 | 11.5 |
| 1 | 91.1 | 2.9 | 93.0 | 0.8 | |
| 5 | 98.8 | 0.9 | 98.6 | 0.6 | |
| 10 | 99.9 | 0.6 | 99.8 | 0.2 | |
| 25 | 98.5 | 2.0 | 98.4 | 2.7 | |
| 50 | 101.0 | 0.2 | 100.7 | 0.4 | |
| Inter-Day | 0.5 | 92.7 | 2.6 | 90.6 | 9.6 |
| 1 | 92.9 | 0.9 | 92.7 | 0.4 | |
| 5 | 98.4 | 0.4 | 98.6 | 0.7 | |
| 10 | 99.7 | 0.3 | 100.0 | 0.5 | |
| 25 | 94.8 | 3.4 | 96.1 | 0.3 | |
| 50 | 100.6 | 0.3 | 101.1 | 0.1 | |
| Real Sample | Manufacturer | Claimed Amount (mg) | Actual Amount (mg) * | % of Labelled Claim |
|---|---|---|---|---|
| Turmeric Capsules | Futurebiotics®, Hauppauge, NY 11788, USA | 500 | 494.15 ± 3.58 | 98.83 |
| Ultrazole | Riyadh Pharma, Saudi Arabia | 30 | 28.95 ± 2.11 | 96.50 |
| Formulation Code | Compositions | Droplet Size (nm) | PDI | Zeta Potential (mV) | SNEDDS | Calculated Solubility (mg/g) | |
|---|---|---|---|---|---|---|---|
| CUR | LNS | ||||||
| F1 | ZRO:I988 (7:3)/KrEL [1:1] | 158.1 ± 18 | 0.44 ± 0.05 | −19.2 ± 1.2 | √ Hazy | 37.8 ± 3.5 | 13.3 ± 0.9 |
| F2 | BSO:I988 (7:3)/KrEL [1:1] | 13.8 ± 0.2 | 0.12 ± 0.02 | −21.3 ± 0.6 | √ Transparent | 23.2 ± 2.3 | 10.2 ± 0.3 |
| Parameter | Uncured NUS2 | Cured NUS2 |
|---|---|---|
| BET Surface Area (m²/g) | 399.2 | 286.4 |
| Micropore area (%) * | 6% | 3% |
| External surface area (%) * | 94% | 97% |
| Pore volume (cm³/g) ** | 1.82 | 1.50 |
| Pore size (nm) *** | 18.3 | 21.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshadidi, A.; Shahba, A.A.-W.; Sales, I.; Rashid, M.A.; Kazi, M. Combined Curcumin and Lansoprazole-Loaded Bioactive Solid Self-Nanoemulsifying Drug Delivery Systems (Bio-SSNEDDS). Pharmaceutics 2022, 14, 2. https://doi.org/10.3390/pharmaceutics14010002
Alshadidi A, Shahba AA-W, Sales I, Rashid MA, Kazi M. Combined Curcumin and Lansoprazole-Loaded Bioactive Solid Self-Nanoemulsifying Drug Delivery Systems (Bio-SSNEDDS). Pharmaceutics. 2022; 14(1):2. https://doi.org/10.3390/pharmaceutics14010002
Chicago/Turabian StyleAlshadidi, Abdulrahman, Ahmad Abdul-Wahhab Shahba, Ibrahim Sales, Md Abdur Rashid, and Mohsin Kazi. 2022. "Combined Curcumin and Lansoprazole-Loaded Bioactive Solid Self-Nanoemulsifying Drug Delivery Systems (Bio-SSNEDDS)" Pharmaceutics 14, no. 1: 2. https://doi.org/10.3390/pharmaceutics14010002