212Pb: Production Approaches and Targeted Therapy Applications
Abstract
:1. Introduction
- -
- Implementation of an automated module for radiopharmaceutical synthesis to perform all the operations necessary to provide the medical personnel with the synthesized preparation directly in a medical institution with a minimum expenditure of time;
- -
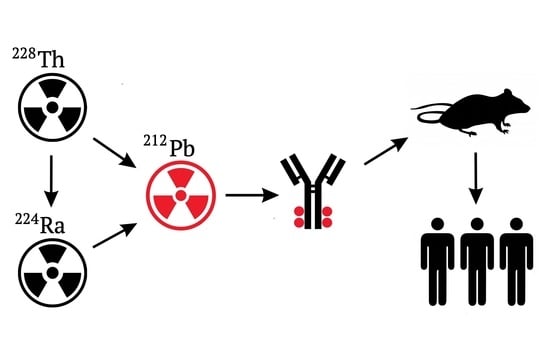
- In vivo generators, which assume application of such radionuclides during labeling of bioconjugates, would generate the required daughter nuclide after introduction into the patient. The radionuclide of this kind for therapeutic 212Bi is 212Pb (T1/2 = 10.64 h) β-emitter. 212Pb is of definite interest because its daughter nuclides (212Bi and 212Po) undergo α-decay, which allows us to view 212Pb as an in vivo generator of α-particles emitters. The radionuclide 212Pb belongs to a radioactive series of long-lived parent 228Th (T1/2 = 1.9 year); see Figure 1.
2. Primary Production Techniques from the Beginning to the Present
3. 212Pb Radiochemistry and Applications
3.1. Chemical Characteristics
3.2. Ligands and Other Carriers for Pb2+
| Conjugate | T °C, Duration | Purification from Free Pb2+ | References |
|---|---|---|---|
| DOTA–AE1 | 35 °C, 45 min | EDTA–SE HPLC | [65] |
| TCMC–trastuzumab | 37 °C, 30 min | EDTA–SE HPLC | [66,67,68] |
| 37 °C, 1 h | [69] | ||
| TCMC–cetuximab | 37 °C, 1 h | EDTA–SE HPLC | [70] |
| TCMC–PSMA | 95 °C, 5 min | - | [71] |
| TCMC–VCAM1 | 37 °C, 30 min | - | [36] |
| TCMC–CC49 (mAb) | 37 °C, 30 min | EDTA–SE HPLC | [64] |
| TCMC–daratumumab | 37 °C, 15 min | - | [72] |
| TCMC–TATE (DOTAMTATE) | 50 °C, 10 min | - | [73] |
| DOTA–CC49 (mAb) | 37 °C, 30 min | EDTA–SE HPLC | [64] |
| DOTA–Re(Arg11)CCMSH (mAb) | 75 °C, 45 min | RP–HPLC | [24,74] |
| DOTATOC | 85 °C, 45 min | - | [19] |
| DOTA–103A (mAb) | 37 °C 30 min | EDTA–SE HPLC | [75] |
| DOTA–biotin | 80 °C 30 min | - | [76] |
3.3. Preclinical, Animal Studies
3.4. Clinical Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DOTMP | 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetramethylenephosphonic acid |
| TCMC/DOTAM | 2-[4,7,10-tris(2-amino-2-oxoethyl)-1,4,7,10-tetrazacyclododec-1-yl]acetamide |
| DOTP | 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetra(methylene phosphonic) acid |
| BAPTA | 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid |
| cyclen | 1,4,7,10-tetraazacyclododecane |
| DOTA | 2,2′,2″,2‴-(1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic acid |
| NOTA | 2,2′,2″-(1,4,7-triazacyclononane-1,4,7-triyl)triacetic acid |
| HEHA | 1,4,7,10,13,16-hexaazacyclooctadecane-N,N′,N″,N‴,N⁗,N″‴-hexaacetic acid |
| TETA | 1,4,8,11-Tetraazacyclotetradecane-1,4,8,11-tetraacetic acid |
| EDTMP | {Ethane-1,2-diylbis[nitrilobis(methylene)]}tetrakis(phosphonic acid) |
| NETA | {4-[2-(bis-carboxy-methylamino)-5-(4-nitrophenyl)pentyl]-7-carbo-xymethyl-[1,4,7]triazanonan-1-yl} acetic acid |
| p-SCN-Bn-TCMC | S-2-(4-Isothiocyanatobenzyl)-1,4,7,10-tetraaza-1,4,7,10-tetra(2-carbamoylmethyl)cyclododecane |
| DO3AM | 2,2′,2″-(1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetamide |
| DOTA-Re(Arg11)CCMSH | DOTA-Re(Arg11)(Cys3,4,10,d-Phe7)-αMSH3-13 |
| PEPA | 1,4,7,10,13-pentaazacyclopentadecane-N,N′,N″,N‴,N⁗-pentaacetic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| CHX-A″-DTPA | [(R)-2-Amino-3-(4-isothiocyanatophenyl)propyl]-trans-(S,S)-cyclohexane-1,2-diamine-pentaacetic acid |
| ACAC | Acetylacetone (Pentane-2,4-dione) |
| en | Ethylenediamine (Ethane-1,2-diamine) |
| C-NE3TA | 4-carboxymethyl-7-[2-(carboxymethyl-amino)-3-(4-nitro-phenyl)-propyl]-[1,4,7]triazonan-1-yl-acetic acid |
| DTPA | diethylenetriaminepentaacetic acid |
| AZEP-DTPA | cis-{2,7-bis-[bis-carboxymethyl-amino)-methyl]-azepan-1-yl}-acetic acid |
| NPTA | [{4-carboxymethyl-7-[2-(carboxymethylamino)-ethyl]-perhydro-1,4,7-triazonin-1-yl}-acetic acid |
| NETA | {4-[2-(bis(carboxymethyl)amino)ethyl]-7- carboxymethyl [1,4,7]triazonon-1-yl}-acetic acid |
| PIP-DTPA | cis-2,6-bis[N,N-bis(carboxymethyl)aminomethyl]-1-piperidineacetic acid |
| p-SCN-Bz-DOTA-GA | 2,2′,2″-(10-(1-carboxy-4-((4-isothiocyanatobenzyl)amino)-4-oxobutyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid |
| EEDTA | Oxybis(ethylenenitrilo)tetraacetic acid (1,7-diaza-4-oxaheptane-l,l,7,7-tetra-acetic acid) |
| EGTA | ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (3,12-Bis(carboxymethyl)-6,9-dioxa-3,12-diazatetradecane-1,14-dioic acid) |
| L1 | 5-[2-(N-2-fluorenyl)ethylamino]-2,5,8-triaza [9]-2,6-pyridinophane |
| THP-cyclen | 1,1′,1″,1‴-(1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetrayl) tetrakis(propan-2-ol) acid |
| VCAM1 | vascular cell adhesion molecule 1 |
| LP | Lone electron pair |
| REE | Rare earth elements |
| mAb | Monoclonal antibody |
| I.P. | intraperitoneal |
| I.V. | intravenous |
| RIT | Radioimmunotherapy |
References
- Öberg, K.; Kvols, L.; Caplin, M.; Fave, G.D.; de Herder, W.; Rindi, G.; Ruszniewski, P.; Woltering, E.A.; Wiedenmann, B. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann. Oncol. 2004, 15, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, H. Somatostatin receptor based imaging and radionuclide therapy. BioMed Res. Int. 2015, 2015, e917968. [Google Scholar] [CrossRef]
- Brechbiel, M.W. Targeted α-therapy: Past, present, future? Dalton Trans. 2007, 43, 4918–4928. [Google Scholar] [CrossRef] [Green Version]
- Yong, K.; Brechbiel, M.W. Towards translation of 212Pb as a clinical therapeutic; Getting the lead in! Dalton Trans. 2011, 40, 6068–6076. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.N.; Moorbath, S. The diffusion of thoron in solids. Part II—The emanating power of barium salts of the fatty acids. Trans. Faraday Soc. 1951, 47, 1064–1072. [Google Scholar] [CrossRef]
- Hursh, J.B.; Lovaas, A.I. Preparation of a dry 228Th source of thoron. J. Inorg. Nucl. Chem. 1967, 29, 599–600. [Google Scholar] [CrossRef]
- Wahl, A.C.; Daniels, W.R. Emanating power of barium stearate for 3.9-second actinon (219Rn). J. Inorg. Nucl. Chem. 1958, 6, 278–287. [Google Scholar] [CrossRef]
- Porstendörfer, J.; Röbig, G.; Ahmed, A. Experimental determination of the attachment coefficients of atoms and ions on monodisperse aerosols. J. Aerosol Sci. 1979, 10, 21–28. [Google Scholar] [CrossRef]
- Porstendorfer, J.; Mercer, T.T. Influence of electric charge and humidity upon the diffusion coefficient of radon decay products. Health Phys. 1979, 37, 191–199. [Google Scholar] [CrossRef]
- Raghunath, B.; Kotrappa, P. Diffusion coefficients of decay products of radon and thoron. J. Aerosol Sci. 1979, 10, 133–138. [Google Scholar] [CrossRef]
- Morimoto, E.M.; Kahn, M. Preparation of carrier-free lead-212 (Thorium B). J. Chem. Educ. 1959, 36, 296. [Google Scholar] [CrossRef]
- Hashimoto, T.; Komatsu, S.; Kido, K.; Sotobayashi, T. Elution behaviour of alpha-recoil atoms into etchant and ovservation of their tracks on the mica surface. Nucl. Instrum. Methods 1980, 178, 437–442. [Google Scholar] [CrossRef]
- Bartoś, B.; Lyczko, K.; Kasperek, A.; Krajewski, S.; Bilewicz, A. Search of ligands suitable for 212Pb/212Bi in vivo generators. J. Radioanal. Nucl. Chem. 2013, 295, 205–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzadeh, S. Generator-produced alpha-emitters. Appl. Radiat. Isot. 1998, 49, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Šebesta, F.; Starý, J. A generator for preparation of carrier-free224Ra. J. Radioanal. Chem. 1974, 21, 151–155. [Google Scholar] [CrossRef]
- Zucchini, G.L.; Friedman, A.M. Isotopic generator for 212Pb and 212Bi. Int. J. Nucl. Med. Biol. 1982, 9, 83–84. [Google Scholar] [CrossRef]
- Atcher, R.W.; Friedman, A.M.; Hines, J.J. An improved generator for the production of 212Pb and 212Bi from 224Ra. Int. J. Radiat. Appl. Instrum. Part A Appl. Radiat. Isot. 1988, 39, 283–286. [Google Scholar] [CrossRef]
- Stenberg, V.Y.; Larsen, R.H.; Ma, L.W.; Peng, Q.; Juzenas, P.; Bruland, Ø.S.; Juzeniene, A. Evaluation of the psma-binding ligand212pb-ng001 in multicellular tumour spheroid and mouse models of prostate cancer. Int. J. Mol. Sci. 2021, 22, 4815. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Quinn, T.P.; Lee, D.; Liu, D.; Kunkel, F.; Zimmerman, B.E.; McAlister, D.; Olewein, K.; Menda, Y.; et al. Automated cassette-based production of high specific activity [203/212 Pb] peptide-based theranostic radiopharmaceuticals for image-guided radionuclide therapy for cancer. Appl. Radiat. Isot. 2017, 127, 52–60. [Google Scholar] [CrossRef]
- Yong, K.J.; Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W. 212 Pb-radioimmunotherapy potentiates paclitaxel-induced cell killing efficacy by perturbing the mitotic spindle checkpoint. Br. J. Cancer 2013, 108, 2013–2020. [Google Scholar] [CrossRef] [Green Version]
- Kasten, B.B.; Azure, M.T.; Schoeb, T.R.; Fisher, D.R.; Zinn, K.R. Imaging, biodistribution, and toxicology evaluation of 212Pb-TCMC-trastuzumab in nonhuman primates. Nucl. Med. Biol. 2016, 43, 391–396. [Google Scholar] [CrossRef]
- Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W. Bench to bedside: Stability studies of GMP produced trastuzumab-TCMC in support of a clinical trial. Pharmaceuticals 2015, 8, 435–454. [Google Scholar] [CrossRef] [Green Version]
- Schneider, N.R.; Lobaugh, M.; Tan, Z.; Sandwall, P.; Chen, P.; Glover, S.E.; Cui, L.; Murry, M.; Dong, Z.; Torgue, J.; et al. Biodistribution of 212Pb conjugated trastuzumab in mice. J. Radioanal. Nucl. Chem. 2013, 296, 75–81. [Google Scholar] [CrossRef]
- Miao, Y.; Hylarides, M.; Fisher, D.R.; Shelton, T.; Moore, H.; Wester, D.W.; Fritzberg, A.R.; Winkelmann, C.T.; Hoffman, T.; Quinn, T.P. Melanoma therapy via peptide-targeted α-radiation. Clin. Cancer Res. 2005, 11, 5616–5621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenberg, V.Y.; Juzeniene, A.; Bruland, Ø.S.; Larsen, R.H. In situ Generated 212 Pb-PSMA Ligand in a 224 Ra-Solution for Dual Targeting of Prostate Cancer Sclerotic Stroma and PSMA-positive Cells. Curr. Radiopharm. 2020, 13, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Kasten, B.B.; Gangrade, A.; Kim, H.; Fan, J.; Ferrone, S.; Ferrone, C.R.; Zinn, K.R.; Buchsbaum, D.J. 212Pb-labeled B7-H3-targeting antibody for pancreatic cancer therapy in mouse models. Nucl. Med. Biol. 2018, 58, 67–73. [Google Scholar] [CrossRef]
- Shah, M.A.; Zhang, X.; Rossin, R.; Robillard, M.S.; Fisher, D.R.; Bueltmann, T.; Hoeben, F.J.M.; Quinn, T.P. Metal-Free Cycloaddition Chemistry Driven Pretargeted Radioimmunotherapy Using α-Particle Radiation. Bioconjug. Chem. 2017, 28, 3007–3015. [Google Scholar] [CrossRef]
- Saidi, A.; Maaland, A.; Torgue, J.; Heyerdahl, H.; Dahle, J. Targeted Alpha Therapy with 212Pb-NNV003 for the Treatment of CD37 Positive B-Cell Chronic Lymphocytic Leukemia (CLL) and Non-Hodgkin Lymphoma (NHL). Blood 2018, 132, 4422. [Google Scholar] [CrossRef]
- Howell, R.W.; Azure, M.T.; Narra, V.R.; Rao, D.V. Relative Biological Effectiveness of Alpha-Particle Emitters In Vivo at Low Doses. Radiat. Res. 1994, 137, 352. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.P.; Bond, A.H. Purification of radionuclides for nuclear medicine: The multicolumn selectivity inversion generator concept. Czechoslov. J. Phys. 2003, 53, A713–A716. [Google Scholar] [CrossRef]
- McAlister, D.R.; Horwitz, E.P. Chromatographic generator systems for the actinides and natural decay series elements. Radiochim. Acta 2011, 99, 151–159. [Google Scholar] [CrossRef]
- Narbutt, J.; Bilewicz, A. Gamma emitting radiotracers 224Ra, 212Pb and 212Bi from natural thorium. Appl. Radiat. Isot. 1998, 49, 89–91. [Google Scholar] [CrossRef]
- Boll, R.A.; Malkemus, D.; Mirzadeh, S. Production of actinium-225 for alpha particle mediated radioimmunotherapy. Appl. Radiat. Isot. 2008, 62, 667–679. [Google Scholar] [CrossRef]
- Diener, M.D.; Afford, J.M.; Kennel, S.J.; Mirzadeh, S. 212Pb@C60 and its water-soluble derivatives: Synthesis, stability, and suitability for radioimmunotherapy. J. Am. Chem. Soc. 2007, 129, 5131–5138. [Google Scholar] [CrossRef]
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, F.M.; Fassbender, M.E.; Simon, S.; Birnbaum, E.R.; Atcher, R.W.; John, K.D.; Rixe, O.; et al. Alpha-Emitters and Targeted Alpha Therapy in Oncology: From Basic Science to Clinical Investigations. Target. Oncol. 2018, 13, 189–203. [Google Scholar] [CrossRef]
- Corroyer-Dulmont, A.; Valable, S.; Falzone, N.; Frelin-Labalme, A.-M.M.; Tietz, O.; Toutain, J.; Soto, M.S.; Divoux, D.; Chazalviel, L.; Pérès, E.A.; et al. VCAM-1 targeted alpha-particle therapy for early brain metastases. Neuro-Oncol. 2020, 22, 357–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arazi, L.; Cooks, T.; Schmidt, M.; Keisari, Y.; Kelson, I. Treatment of solid tumors by interstitial release of recoiling short-lived alpha emitters. Phys. Med. Biol. 2007, 52, 5025–5042. [Google Scholar] [CrossRef]
- Hassfjell, S.P.; Hoff, P. A generator for production of 212Pb and 212Bi. Appl. Radiat. Isot. 1994, 45, 1021–1025. [Google Scholar] [CrossRef]
- Hassfjell, S. A 212Pb generator based on a 228Th source. Appl. Radiat. Isot. 2001, 55, 433–439. [Google Scholar] [CrossRef]
- Boldyrev, P.P.; Egorova, B.V.; Kokov, K.V.; Perminov, Y.A.; Proshin, M.A.; Chuvilin, D.Y. Physical and chemical processes on the 212Pb radionuclide production for nuclear medicine. J. Phys. Conf. Ser. 2018, 1099, 012003. [Google Scholar] [CrossRef]
- Chuvilin, D.; Kokov, K.; Egorova, B.; Makoveeva, K.; Perminov, Y.; Proshin, M. Synthesis and Investigation of a Preparation Based on 212 Pb-Labeled DOTATATE Synthetic Peptide for Therapy of Neuroendocrine Tumors. In Proceedings of the V International Conference for Young Scientists, Post-Graduate Students and Students “Isotopes: Technologies, Materials and Application”, Tomsk, Russia, 18–23 November 2018; AIP Publishing: Melville, NY, USA, 2019; Volume 2101, pp. 1–8. [Google Scholar] [CrossRef]
- Kokov, K.; Demchenko, A.; Egorova, B.; Larkin, A.; Lyundup, A.; Makoveeva, K.; Moiseeva, A.; Panchenko, V.; Proshin, M.; Reshetov, I.; et al. Production and Investigation of Radiopharmaceutical Nanoconstruction [212Pb] DOTATATE for Therapy of Malignant Neoplasms. J. Surf. Investig. 2020, 14, S99–S104. [Google Scholar] [CrossRef]
- Pankratov, A.A.; Nemtsova, E.R.; Plyutinskaya, A.D.; Vorontsova, M.S.; Chuvilin, D.Y.; Egorova, B.V.; Kokov, K.V.; Deev, S.M.; Lebedenko, E.N.; Proshkina, G.M.; et al. Specific Cytotoxicity of Targeted 177Lu and 212Pb-Based Radiopharmaceuticals. Bull. Exp. Biol. Med. 2021, 171, 627–632. [Google Scholar] [CrossRef]
- Dougherty, T.F.; Stover, B.J.; Dougherty, J.H.; Jee, W.S.S.; Mays, C.W.; Rehfeld, C.E.; Christensen, W.R.; Goldthorpe, H.C. Studies of the Biological Effects of Ra 226, Pu 239, Ra 228 (MsTh 1), Th 228 (RdTh), and Sr 90 in Adult Beagles. Radiat. Res. 1962, 17, 625. [Google Scholar] [CrossRef]
- Lloyd, R.D.; Jones, C.W.; Mays, C.W.; Atherton, D.R.; Bruenger, F.W.; Taylor, G.N. 228 Th Retention and Dosimetry in Beagles. Radiat. Res. 1984, 98, 614. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Martell, A.E.; Hancock, R.D. Metal Complexes in Aqueous Solutions; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1996. [Google Scholar] [CrossRef]
- Hancock, R.D.; Reibenspies, J.H.; Maumela, H. Structural Effects of the Lone Pair on Lead(II), and Parallels with the Coordination Geometry of Mercury(II). Does the Lone Pair on Lead(II) Form H-Bonds? Structures of the Lead(II) and Mercury(II) Complexes of the Pendant-Donor Macrocycle DOTAM (1, 4, 7, 10-tetrakis (carbamoylmethyl)-1, 4, 7, 10-tetraazacyclododecane). Inorg. Chem. 2004, 43, 2981–2987. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.D.; Shaikjee, M.S.; Dobson, S.M.; Boeyens, J.C.A. The Stereochemical activity or non-activity of the “Inert” pair of electrons on lead(II) in relation to its complex stability and structural properties. Some considerations in ligand design. Inorganica Chim. Acta 1988, 154, 229–238. [Google Scholar] [CrossRef]
- Cuenot, F.; Meyer, M.; Espinosa, E.; Bucaille, A.; Burgat, R.; Guilard, R.; Marichal-Westrich, C. New insights into the complexation of lead(II) by 1,4,7,10-tetrakis(carbamoylmethyl)-1,4,7,10-tetraazacyclododecane (DOTAM): Structural, thermodynamic, and kinetic studies. Eur. J. Inorg. Chem. 2007, 2008, 267–283. [Google Scholar] [CrossRef]
- Nugent, J.W.; Lee, H.S.; Reibenspies, J.H.; Hancock, R.D. Spectroscopic, structural, and thermodynamic aspects of the stereochemically active lone pair on lead(II): Structure of the lead(II) dota complex. Polyhedron 2015, 91, 120–127. [Google Scholar] [CrossRef]
- Liberato, A.; Aguinaco, A.; Clares, M.P.; Delgado-Pinar, E.; Pitarch-Jarque, J.; Blasco, S.; Basallote, M.G.; García-España, E.; Verdejo, B. Pb2+ complexes of small-cavity azamacrocyclic ligands: Thermodynamic and kinetic studies. Dalton Trans. 2017, 46, 6645–6653. [Google Scholar] [CrossRef]
- Moncomble, A.; Cornard, J.P.; Meyer, M. A quantum chemistry evaluation of the stereochemical activity of the lone pair in PbII complexes with sequestering ligands. J. Mol. Model. 2017, 23, 24. [Google Scholar] [CrossRef]
- Nazarenko, A.Y.; Rusanov, E.B. Synthesis and crystal structure of lead thiocyanate complexes with 18-crown-6 and two isomers of dicyclohexane-18-crown-6. Polyhedron 1994, 13, 2549–2553. [Google Scholar] [CrossRef]
- Hancock, R.D.; Martell, A.E. Ligand design for selective complexation of metal ions in aqueous solution. Chem. Rev. 1989, 89, 1875–1914. [Google Scholar] [CrossRef]
- Chaves, S.; Delgado, R.; Da Silva, J.J.R.F. The stability of the metal complexes of cyclic tetra-aza tetra-acetic acids. Talanta 1992, 39, 249–254. [Google Scholar] [CrossRef]
- Pippin, C.G.; McMurry, T.J.; Brechbiel, M.W.; McDonald, M.; Lambrecht, R.; Milenic, D.; Roselli, M.; Colcher, D.; Gansow, O.A. Lead(II) complexes of 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetate: Solution chemistry and application to tumor localization with 203Pb labeled monoclonal antibodies. Inorganica Chim. Acta 1995, 239, 43–51. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E. Critical Stability Constants; Springer: Berlin/Heidelberg, Germany, 1976; Volume 6, ISBN 9781461567660. [Google Scholar]
- Zhao, F.; Repo, E.; Yin, D.; Sillanpää, M.E.T. Adsorption of Cd(II) and Pb(II) by a novel EGTA-modified chitosan material: Kinetics and isotherms. J. Colloid Interface Sci. 2013, 409, 174–182. [Google Scholar] [CrossRef]
- Xu, Z.; Jones, M.M. Comparative mobilization of lead by chelating agents. Toxicology 1988, 53, 277–288. [Google Scholar]
- Chong, H.-S.; Milenic, D.E.; Garmestani, K.; Brady, E.D.; Arora, H.; Pfiester, C.; Brechbiel, M.W. In vitro and in vivo evaluation of novel ligands for radioimmunotherapy. Nucl. Med. Biol. 2006, 33, 459–467. [Google Scholar] [CrossRef]
- Edem, P.E.; Fonslet, J.; Kjær, A.; Herth, M.; Severin, G. In vivo radionuclide generators for diagnostics and therapy. Bioinorg. Chem. Appl. 2016, 2016, e6148357. [Google Scholar] [CrossRef]
- Csajbok, E.; Baranyai, Z.; Banyai, I.; Erno, B.; Kiraly, R.; Mueller-Fahrnow, A.; Platzek, J.; Raduechel, B.; Schaefer, M. Equilibrium, 1 H and 13 C NMR Spectroscopy, and X-ray Diffraction Studies on the Complexes Bi (DOTA)—And Bi (DO3A-Bu). Inorg. Chem. 2003, 42, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Chappell, L.L.; Dadachova, E.; Milenic, D.E.; Garmestani, K.; Wu, C.; Brechbiel, M.W. Synthesis, characterization, and evaluation of a novel bifunctional chelating agent for the lead isotopes 203Pb and 212Pb. Nucl. Med. Biol. 2000, 27, 93–100. [Google Scholar] [CrossRef]
- Horak, E.; Hartmann, F.; Garmestani, K.; Wu, C.; Brechbiel, M.; Gansow, O.A.; Landolfi, N.F.; Waldmann, T.A. Radioimmunotherapy targeting of HER2/neu oncoprotein on ovarian tumor using lead-212-DOTA-AE1. J. Nucl. Med. 1997, 38, 1944–1950. [Google Scholar]
- Tan, Z.; Chen, P.; Schneider, N.; Glover, S.; Cui, L.; Torgue, J.; Rixe, O.; Spitz, H.B.; Dong, Z. Significant systemic therapeutic effects of high-LET immunoradiation by 212Pb-trastuzumab against prostatic tumors of androgen-independent human prostate cancer in mice. Int. J. Oncol. 2012, 40, 1881–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baidoo, K.E.; Milenic, D.E.; Brechbiel, M.W. Methodology for labeling proteins and peptides with lead-212 (212Pb). Nucl. Med. Biol. 2013, 40, 592–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milenic, D.E.; Garmestani, K.; Brady, E.D.; Albert, P.S.; Ma, D.; Abdulla, A.; Brechbiel, M.W. α-particle radioimmunotherapy of disseminated peritoneal disease using a 212Pb-labeled radioimmunoconjugate targeting HER2. Cancer Biother. Radiopharm. 2005, 20, 557–568. [Google Scholar] [CrossRef]
- Westrøm, S.; Generalov, R.; Bønsdorff, T.B.; Larsen, R.H. Preparation of 212Pb-labeled monoclonal antibody using a novel 224Ra-based generator solution. Nucl. Med. Biol. 2017, 51, 1–9. [Google Scholar] [CrossRef]
- Milenic, D.E.; Baidoo, K.E.; Kim, Y.S.; Brechbiel, M.W. Evaluation of cetuximab as a candidate for targeted α-particle radiation therapy of HER1-positive disseminated intraperitoneal disease. mAbs 2015, 7, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, J.C.; Schäfer, M.; Bauder-Wüst, U.; Lehnert, W.; Leotta, K.; Morgenstern, A.; Kopka, K.; Haberkorn, U.; Mier, W.; Kratochwil, C. Development and dosimetry of 203 Pb/212 Pb-labelled PSMA ligands: Bringing “the lead” into PSMA-targeted alpha therapy? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Quelven, I.; Monteil, J.; Sage, M.; Saidi, A.; Mounier, J.; Bayout, A.; Garrier, J.; Cogne, M.; Durand-Panteix, S. 212Pb α-radioimmunotherapy targeting CD38 in multiple myeloma: A preclinical study. J. Nucl. Med. 2020, 61, 1058–1065. [Google Scholar] [CrossRef] [Green Version]
- Stallons, T.A.R.; Saidi, A.; Tworowska, I.; Delpassand, E.S.; Torgue, J.J. Preclinical investigation of 212Pb-DOTAMTATE for peptide receptor radionuclide therapy in a neuroendocrine tumor model. Mol. Cancer Ther. 2019, 18, 1012–1021. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Figueroa, S.D.; Fisher, D.R.; Moore, H.A.; Testa, R.F.; Hoffman, T.J.; Quinn, T.P. 203Pb-labeled α-melanocyte-stimulating hormone peptide as an imaging probe for melanoma detection. J. Nucl. Med. 2008, 49, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Ruble, G.; Chuanchu, W.; Squire, R.A.; Gansow, O.A.; Strand, M. The use of 212PB-labeled monoclonal antibody in the treatment of murine erythroleukemia. Int. J. Radiat. Oncol. Biol. Phys. 1996, 34, 609–616. [Google Scholar] [CrossRef]
- Su, F.M.; Beaumier, P.; Axworthy, D.; Atcher, R.; Fritzberg, A. Pretargeted radioimmunotherapy in tumored mice using an in vivo 212Pb/212Bi generator. Nucl. Med. Biol. 2005, 32, 741–747. [Google Scholar] [CrossRef]
- Mirzadeh, S.; Kumar, K.; Gansow, O.A. The Chemical Fate of 212Bi-DOTA Formed β- Decay of 212Pb (DOTA)2-. Radiochim. Acta 1993, 60, 1–10. [Google Scholar] [CrossRef]
- Maaland, A.F.; Saidi, A.; Torgue, J.; Heyerdahl, H.; Stallons, T.A.R.; Kolstad, A.; Dahle, J.; Stallons, T.A.R.; Kolstad, A.; Dahle, J. Targeted alpha therapy for chronic lymphocytic leukaemia and non-Hodgkin’s lymphoma with the anti-CD37 radioimmunoconjugate 212Pb-NNV003. PLoS ONE 2020, 15, e0230526. [Google Scholar] [CrossRef] [Green Version]
- Zaid, N.; Kletting, P.; Winter, G.; Prasad, V.; Beer, A.J.; Glatting, G. A Physiologically Based Pharmacokinetic Model for In Vivo Alpha Particle Generators Targeting Neuroendocrine Tumors in Mice. Pharmaceutics 2021, 13, 2132. [Google Scholar] [CrossRef]
- Stenberg, V.Y.; Juzeniene, A.; Chen, Q.; Yang, X.; Bruland, Ø.S.; Larsen, R.H. Preparation of the alpha-emitting prostate-specific membrane antigen targeted radioligand [212 Pb] Pb-NG001 for prostate cancer. J. Label. Compd. Radiopharm. 2020, 63, 129–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maumela, H.; Hancock, R.D.; Carlton, L.; Reibenspies, J.H.; Wainwright, K.P. The Amide Oxygen as a Donor Group. Metal Ion Complexing Properties of Tetra-N-acetamide Substituted Cyclen: A Crystallographic, NMR, Molecular Mechanics, and Thermodynamic Study. J. Am. Chem. Soc. 1995, 117, 6698–6707. [Google Scholar] [CrossRef]
- Kumar, K.; Magerstaedt, M.; Gansow, O.A. Lead(II) and Bismuth(III) Complexes of the Polyazacycloalkane-N-acetic Acids nota, dota, and teta. J. Chem. Soc. Chem. Commun. 1989, 3, 145–146. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.S.; Song, H.A.; Ma, X.; Milenic, D.E.; Brady, E.D.; Lim, S.; Lee, H.; Baidoo, K.; Cheng, D.; Brechbiel, M.W. Novel bimodal bifunctional ligands for radioimmunotherapy and targeted MRI. Bioconjugate Chem. 2008, 19, 1439–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadachova, E.; Chappell, L.L.; Brechbiel, M.W. Spectrophotometric method for determination of bifunctional macrocyclic ligands in macrocyclic ligand-protein conjugates. Nucl. Med. Biol. 1999, 26, 977–982. [Google Scholar] [CrossRef]
- Hassfjell, S.P.; Bruland, S.; Hoff, P. 212Bi-DOTMP: An alpha particle emitting bone-seeking agent for targeted radiotherapy. Nucl. Med. Biol. 1997, 24, 231–237. [Google Scholar] [CrossRef]
- Le Du, A.; Mougin-Degraef, M.; Botosoa, E.; Rauscher, A.; Chauvet, A.F.; Barbet, J.; Montavon, G. In vivo 212Pb/212Bi generator using indium-DTPA-tagged liposomes. Radiochim. Acta 2011, 99, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Rotmensch, J.; Atcher, R.W.; Hines, J.; Toohill, M.; Herbst, A.L. Comparison of short-lived high-LET α-emitting radionuclides lead-212 and bismuth-212 to low-LET X-rays on ovarian carcinoma. Gynecol. Oncol. 1989, 35, 297–300. [Google Scholar] [CrossRef]
- Rotmensch, J.; Atcher, R.W.; Hines, J.; Grdina, D.; Schwartz, J.S.; Toohill, M.; Herbst, A.L. The development of α-emitting radionuclide lead 212 for the potential treatment of ovarian carcinoma. Am. J. Obstet. Gynecol. 1989, 160, 789–797. [Google Scholar] [CrossRef]
- Rotmensch, J.; Atcher, R.W.; Schlenker, R.; Hines, J.; Grdina, D.; Block, B.S.; Press, M.F.; Herbst, A.L.; Weichselbaum, R.R. The effect of the α-emitting radionuclide lead-212 on human ovarian carcinoma: A potential new form of therapy. Gynecol. Oncol. 1989, 32, 236–239. [Google Scholar] [CrossRef]
- Rosenow, M.K.; Zucchini, G.L.; Bridwell, P.M.; Stuart, F.P.; Friedman, A.M. Properties of Liposomes Containing 212Pb. Int. J. Nucl. Med. Biol. 1983, 10, 189–197. [Google Scholar] [CrossRef]
- Repetto-Llamazares, A.H.V.; Larsen, R.H.; Patzke, S.; Fleten, K.G.; Didierlaurent, D.; Pichard, A.; Pouget, J.P.; Dahle, J. Targeted cancer therapy with a novel anti-CD37 beta-particle emitting radioimmunoconjugate for treatment of non-hodgkin lymphoma. PLoS ONE 2015, 10, e0128816. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milenic, D.E.; Garmestani, K.; Brady, E.D.; Albert, P.S.; Abdulla, A.; Flynn, J.; Brechbiel, M.W. Potentiation of high-LET radiation by gemcitabine: Targeting HER2 with trastuzumab to treat disseminated peritoneal disease. Clin. Cancer Res. 2007, 13, 1926–1935. [Google Scholar] [CrossRef] [Green Version]
- Milenic, D.E.; Garmestani, K.; Brady, E.D.; Baidoo, K.E.; Albert, P.S.; Wong, K.J.; Flynn, J.; Brechbiel, M.W. Multimodality therapy: Potentiation of high linear energy transfer radiation with paclitaxel for the treatment of disseminated peritoneal disease. Clin. Cancer Res. 2008, 14, 5108–5115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudousq, V.; Bobyk, L.; Busson, M.; Garambois, V.; Jarlier, M.; Charalambatou, P.; Pèlegrin, A.; Paillas, S.; Chouin, N.; Quenet, F.; et al. Comparison between Internalizing Anti-HER2 mAbs and Non-Internalizing Anti-CEA mAbs in Alpha-Radioimmunotherapy of Small Volume Peritoneal Carcinomatosis Using 212Pb. PLoS ONE 2013, 8, e69613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, K.J.; Milenic, D.E.; Baidoo, K.E.; Kim, Y.S.; Brechbiel, M.W. Gene expression profiling upon 212Pb-TCMC-trastuzumab treatment in the LS-174T i.p. xenograft model. Cancer Med. 2013, 2, 646–653. [Google Scholar] [CrossRef]
- Yong, K.J.; Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W. Sensitization of tumor to 212Pb radioimmunotherapy by gemcitabine involves initial abrogation of G2 arrest and blocked DNA damage repair by interference with Rad51. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1119–1126. [Google Scholar] [CrossRef] [Green Version]
- Yong, K.J.; Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W. Cell killing mechanisms and impact on gene expression by gemcitabine and 212Pb-trastuzumab treatment in a disseminated i.p. tumor model. PLoS ONE 2016, 11, e0159904. [Google Scholar] [CrossRef]
- Tworowska, I.; Wagh, N.; Delpassand, E.S.; Rojas-Quijan, F.; Jurek, P.; Kiefer, G.E.; Stallons, T.A.; Saidi, A.; Torgue, J. Treatment of Cancer Cells Overexpressing Somatostatin Receptors Using Ocreotide Derivatives Chelated to Radioisotopes. U.S. Patent 16/477,623, 7 November 2019. [Google Scholar]
- Morgenstern, A.; Apostolidis, C.; Bruchertseifer, F. Supply and Clinical Application of Actinium-225 and Bismuth-213. Semin. Nucl. Med. 2020, 50, 119–123. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Minn, I.; Kumar, V.; Josefsson, A.; Lisok, A.; Brummet, M.; Chen, J.; Kiess, A.P.; Baidoo, K.; Brayton, C.; et al. Preclinical evaluation of 203/212Pb-labeled low-molecular-weight compounds for targeted radiopharmaceutical therapy of prostate cancer. J. Nucl. Med. 2020, 61, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Pouget, J.; Ladjohounlou, R.; Pichard, A.; Boudousq, V.; Bobyk, L.; Paillas, S.; Le Blay, M.; Busson, M.; Lozza, C.; Torgue, J.; et al. Dose-effect relationship during alpha-RIT of small volume peritoneal carcinomatosis using 212pb-labeled mabs. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, S191–S192. [Google Scholar]
- Durand-Panteix, S.; Monteil, J.; Sage, M.; Garot, A.; Clavel, M.; Saidi, A.; Torgue, J.; Cogne, M.; Quelven, I. Preclinical study of 212Pb alpha-radioimmunotherapy targeting CD20 in non-Hodgkin lymphoma. Br. J. Cancer 2021, 125, 1657–1665. [Google Scholar] [CrossRef]
- Kasten, B.B.; Arend, R.C.; Katre, A.A.; Kim, H.; Fan, J.; Ferrone, S.; Zinn, K.R.; Buchsbaum, D.J. B7-H3-targeted 212Pb radioimmunotherapy of ovarian cancer in preclinical models. Nucl. Med. Biol. 2017, 47, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Falzone, N.; Ackerman, N.L.; de la Rosales, L.F.; Bernal, M.A.; Liu, X.; Peeters, S.G.J.A.; Soto, M.S.; Corroyer-Dulmont, A.; Bernaudin, M.; Grimoin, E.; et al. Dosimetric evaluation of radionuclides for VCAM-1-targeted radionuclide therapy of early brain metastases. Theranostics 2018, 8, 292–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackerman, N.L.; de la Rosales, L.F.; Falzone, N.; Vallis, K.A.; Bernal, M.A. Targeted alpha therapy with 212Pb or 225Ac: Change in RBE from daughter migration. Phys. Medica 2018, 51, 91–98. [Google Scholar] [CrossRef]
- Li, R.G.; Lindland, K.; Tonstad, S.K.; Bønsdorff, T.B.; Juzeniene, A.; Westrøm, S.; Larsen, R.H. Improved formulation of 224Ra-labeled calcium carbonate microparticles by surface layer encapsulation and addition of EDTMP. Pharmaceutics 2021, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Bernoulli, J.; Suominen, M.; Halleen, J.; Larsen, R.H. Antitumor activity of novel bone-seeking, α-emitting 224 Ra-solution in a breast cancer skeletal metastases model. Anticancer Res. 2018, 38, 1947–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, R.F.; Torgue, J.; Azure, M.T.; Shen, S.; Saddekni, S.; Banaga, E.; Carlise, R.; Bunch, P.; Yoder, D.; Alvarez, R. Pharmacokinetics and imaging of 212Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother. Radiopharm. 2014, 29, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Meredith, R.; Torgue, J.; Shen, S.; Fisher, D.R.; Banaga, E.; Bunch, P.; Morgan, D.; Fan, J.; Straughn, J.M. Dose escalation and dosimetry of first-in-human α radioimmunotherapy with 212Pb-TCMC-trastuzumab. J. Nucl. Med. 2014, 55, 1636–1642. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Frey, E.; Sgouros, G.; Ghaly, M.; Tworowska, I.; Delpassand, E. Development and Validation of Methods for Quantitative In Vivo SPECT of Pb-212. J. Med. Imaging Radiat. Sci. 2019, 50, S104. [Google Scholar] [CrossRef]
- Meredith, R.F.; Torgue, J.J.; Rozgaja, T.A.; Banaga, E.P.; Bunch, P.W.; Alvarez, R.D.; Straughn, J.M.; Dobelbower, M.C.; Lowy, A.M. Safety and outcome measures of first-in-human intraperitoneal α radioimmunotherapy with 212 Pb-TCMC-Trastuzumab. Am. J. Clin. Oncol. 2018, 41, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Delpassand, E.; Tworowska, I.; Shannon, F.; Nunez, R.; Flores, L., II; Muzammil, A.; Stallons, T.; Saidi, A.; Torgue, J. First clinical experience using targeted alpha-emitter therapy with 212Pb-DOTAMTATE (AlphaMedix TM) in patients with SSTR(+) neuroendocrine tumors. J. Nucl. Med. 2019, 60, 559. [Google Scholar]
- Delpassand, E.; Tworowska, I.; Torgue, J.; Hurt, J.; Nuñez, R.; Esfandiari, R. 212Pb-AlphaMedixTM Targeted Alpha Therapy (TAT): A Potential Breakthrough in Treatment of Metastatic SSTR Expressing NET. In Proceedings of the NANETS 2020 Symposium Abstracts, Montreal, QC, Canada, 1–3 October 2020; North American Neuroendocrine Tumor Society: Albany, NY, USA, 2020; p. 193. [Google Scholar]
- Mcneil, B.L.; Robertson, A.K.H.; Fu, W.; Yang, H.; Hoeht, C.; Ramogida, C.F.; Schaffer, P. Production, Purification, and Radiolabeling of the 203Pb/212Pb Theranostic Pair. EJNMMI Radiopharm. Chem. 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed]




| Compound | Pb–DOTA [51] | Pb–TCMC [48] | Pb–TCMC [50] | Pb–THP–Cyclen [55] | Pb–L3 [52] | Pb–L1 [52] |
|---|---|---|---|---|---|---|
| Pb–N1 | 2.687 | 2.654 | 2.617 | 2.64 | 2.4 | 2.53 |
| Pb–N2 | 2.638 | 2.61 | 2.625 | 2.64 | 2.524 | 2.64 |
| Pb–N3 | 2.66 | 2.641 | 2.628 | 2.64 | 2.42 | 2.51 |
| Pb–N4 | 2.676 | 2.629 | 2.63 | 2.64 | 2.502 | 2.59 |
| Pb–O1 | 2.796 | 2.777 | 2.657 | 2.71 | 3.11 | 3.03 |
| Pb–O2 | 2.856 | 2.902 | 2.785 | 2.71 | 3.22 | 3.09 |
| Pb–O3 | 2.827 | 2.667 | 2.756 | 2.78 | 2.96 | - |
| Pb–O4 | 2.609 | 2.77 | 2.819 | 2.78 | 3.11 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokov, K.V.; Egorova, B.V.; German, M.N.; Klabukov, I.D.; Krasheninnikov, M.E.; Larkin-Kondrov, A.A.; Makoveeva, K.A.; Ovchinnikov, M.V.; Sidorova, M.V.; Chuvilin, D.Y. 212Pb: Production Approaches and Targeted Therapy Applications. Pharmaceutics 2022, 14, 189. https://doi.org/10.3390/pharmaceutics14010189
Kokov KV, Egorova BV, German MN, Klabukov ID, Krasheninnikov ME, Larkin-Kondrov AA, Makoveeva KA, Ovchinnikov MV, Sidorova MV, Chuvilin DY. 212Pb: Production Approaches and Targeted Therapy Applications. Pharmaceutics. 2022; 14(1):189. https://doi.org/10.3390/pharmaceutics14010189
Chicago/Turabian StyleKokov, Konstantin V., Bayirta V. Egorova, Marina N. German, Ilya D. Klabukov, Michael E. Krasheninnikov, Antonius A. Larkin-Kondrov, Kseniya A. Makoveeva, Michael V. Ovchinnikov, Maria V. Sidorova, and Dmitry Y. Chuvilin. 2022. "212Pb: Production Approaches and Targeted Therapy Applications" Pharmaceutics 14, no. 1: 189. https://doi.org/10.3390/pharmaceutics14010189