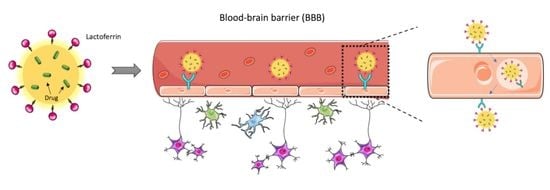
Formulation, Characterization, and Cytotoxicity Evaluation of Lactoferrin Functionalized Lipid Nanoparticles for Riluzole Delivery to the Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Lipid Nanoparticles
2.3. Functionalization of NLC with Lactoferrin
2.4. Physicochemical Characterization and Stability of NLC
2.4.1. Particle Size, PDI, and ZP Analysis
2.4.2. Encapsulation Efficiency (EE)
2.5. Transmission Electron Microscopy (TEM)
2.6. In Vitro Drug Release Studies
2.7. Lactoferrin Conjugation Efficiency
2.8. Fourier Transform Infrared (FTIR) Spectroscopy
2.9. Differential Scanning Calorimetry (DSC)
2.10. Powder X-ray Diffraction (PXRD)
2.11. Cell Culture
2.12. MTT Cytotoxicity Assay
3. Results and Discussion
3.1. Physicochemical Characterization and Stability Studies
3.2. In Vitro Release Studies
3.3. Morphology Determination
3.4. Lactoferrin Conjugation Efficiency
3.5. FTIR Spectroscopy
3.6. DSC
3.7. PXRD Analysis
3.8. Cell Viability Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norris, S.P.; Likanje, M.-F.N.; Andrews, J.A. Amyotrophic lateral sclerosis: Update on clinical management. Curr. Opin. Neurol. 2020, 33, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.E.; Patani, R. The microglial component of amyotrophic lateral sclerosis. Brain 2020, 143, 3526–3539. [Google Scholar] [CrossRef]
- Goyal, N.A.; Berry, J.D.; Windebank, A.; Staff, N.P.; Maragakis, N.J.; van den Berg, L.H.; Genge, A.; Miller, R.; Baloh, R.H.; Kern, R.; et al. Addressing heterogeneity in amyotrophic lateral sclerosis CLINICAL TRIALS. Muscle Nerve 2020, 62, 156–166. [Google Scholar] [CrossRef] [Green Version]
- ALS Association. FDA-Approved Drugs. Available online: https://www.als.org/navigating-als/living-with-als/fda-approved-drugs (accessed on 25 November 2021).
- Wang, G.Y.; Rayner, S.L.; Chung, R.; Shi, B.Y.; Liang, X.J. Advances in nanotechnology-based strategies for the treatments of amyotrophic lateral sclerosis. Mater. Today Bio 2020, 6, 100055. [Google Scholar] [CrossRef]
- Schönfelder, E.; Osmanovic, A.; Müschen, L.H.; Petri, S.; Schreiber-Katz, O. Costs of illness in amyotrophic lateral sclerosis (ALS): A cross-sectional survey in Germany. Orphanet J. Rare Dis. 2020, 15, 149. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Current insights on lipid nanocarrier-assisted drug delivery in the treatment of neurodegenerative diseases. Eur. J. Pharm. Biopharm. 2020, 149, 192–217. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. HEALEY ALS Platform Trial—Master Protocol. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04297683?term=CNM-Au8&draw=2&rank=10 (accessed on 25 November 2021).
- ClinicalTrials.gov. HEALEY ALS Platform Trial—Regimen C CNM-Au. Available online: https://clinicaltrials.gov/ct2/show/NCT04414345?term=CNM-Au8&draw=2&rank=1 (accessed on 25 November 2021).
- ClinicalTrials.gov. 31P-MRS Imaging to Assess the Effects of CNM-Au8 on Impaired Neuronal Redox State in Amyotrophic Lateral Sclerosis (REPAIR-ALS) (REPAIR-ALS). Available online: https://clinicaltrials.gov/ct2/show/NCT03843710?term=CNM-Au8&draw=2&rank=5 (accessed on 25 November 2021).
- ClinicalTrials.gov. Intermediate Expanded Access Protocol for ALS. Available online: https://clinicaltrials.gov/ct2/show/NCT04081714?term=CNM-Au8&draw=2&rank=7 (accessed on 25 November 2021).
- ClinicalTrials.gov. Therapeutic Nanocatalysis to Slow Disease Progression of Amyotrophic Lateral Sclerosis (ALS) (RES-CUE-ALS). Available online: https://clinicaltrials.gov/ct2/show/NCT04098406?term=CNM-Au8&draw=2&rank=8 (accessed on 25 November 2021).
- Wiley, N.J.; Madhankumar, A.B.; Mitchell, R.M.; Neely, E.B.; Rizk, E.; Douds, G.L.; Simmons, Z.; Connor, J.R. Lipopolysaccharide Modified Liposomes for Amyotropic Lateral Sclerosis Therapy: Efficacy in SOD1 Mouse Model. Adv. Nanoparticles 2012, 1, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Ferrill, L.; Gallant, L.; McGillicuddy, S.; Fernandes, T.; Schields, N.; Bai, S. Verapamil and riluzole cocktail liposomes overcome pharmacoresistance by inhibiting P-glycoprotein in brain endothelial and astrocyte cells: A potent approach to treat amyotrophic lateral sclerosis. Eur. J. Pharm. Sci. 2018, 120, 30–39. [Google Scholar] [CrossRef]
- Bondì, M.L.; Craparo, E.F.; Giammona, G.; Drago, F. Brain-targeted solid lipid nanoparticles containing riluzole: Preparation, characterization and biodistribution. Nanomedicine 2010, 5, 25–32. [Google Scholar] [CrossRef]
- Parikh, R.H.; Patel, R.J. Nanoemulsions for Intranasal Delivery of Riluzole to Improve Brain Bioavailability: Formulation Development and Pharmacokinetic Studies. Curr. Drug Deliv. 2016, 13, 1130–1143. [Google Scholar] [CrossRef]
- Chen, L.; Watson, C.; Morsch, M.; Cole, N.J.; Chung, R.S.; Saunders, D.N.; Yerbury, J.J.; Vine, K.L. Improving the Delivery of SOD1 Antisense Oligonucleotides to Motor Neurons Using Calcium Phosphate-Lipid Nanoparticles. Front. Neurosci. 2017, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-Q.; Ke, W.-L.; Qu, Y.-H.; Zhu, J.-H.; Pei, Y.-Y.; Jiang, C. Characterization of lactoferrin receptor in brain endothelial capillary cells and mouse brain. J. Biomed. Sci. 2006, 14, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef]
- Singh, I.; Swami, R.; Pooja, D.; Jeengar, M.K.; Khan, W.; Sistla, R. Lactoferrin bioconjugated solid lipid nanoparticles: A new drug delivery system for potential brain targeting. J. Drug Target. 2016, 24, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Ahsan, S.M.; Kumar, J.M.; Kondapi, A.K.; Rao, N.M. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, R.G.R.; Granja, A.; Loureiro, J.A.; Pereira, M.C.; Pinheiro, M.; Neves, A.R.; Reis, S. RVG29-Functionalized Lipid Nanoparticles for Quercetin Brain Delivery and Alzheimer’s Disease. Pharm. Res. 2020, 37, 1–12. [Google Scholar] [CrossRef]
- Pinheiro, R.G.R.; Granja, A.; Loureiro, J.; Pereira, M.C.; Pinheiro, M.; Neves, A.R.; Reis, S. Quercetin lipid nanoparticles functionalized with transferrin for Alzheimer’s disease. Eur. J. Pharm. Sci. 2020, 148, 105314. [Google Scholar] [CrossRef]
- Khan, S.A.; Rehman, S.; Nabi, B.; Iqubal, A.; Nehal, N.; Fahmy, U.A.; Kotta, S.; Baboota, S.; Md, S.; Ali, J. Boosting the Brain Delivery of Atazanavir through Nanostructured Lipid Carrier-Based Approach for Mitigating NeuroAIDS. Pharmaceutics 2020, 12, 1059. [Google Scholar] [CrossRef]
- Vieira, R.; Severino, P.; Nalone, L.A.; Souto, S.B.; Silva, A.M.; Lucarini, M.; Durazzo, A.; Santini, A.; Souto, E.B. Sucupira Oil-Loaded Nanostructured Lipid Carriers (NLC): Lipid Screening, Factorial Design, Release Profile, and Cytotoxicity. Molecules 2020, 25, 685. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Mei, D.; Huo, Y.; Mao, S. Effect of polysorbate 80 on the intranasal absorption and brain distribution of tetramethylpyrazine phosphate in rats. Drug Deliv. Transl. Res. 2018, 9, 311–318. [Google Scholar] [CrossRef]
- Jahan, S.T.; Sadat, S.M.A.; Walliser, M.; Haddadi, A. Targeted Therapeutic Nanoparticles: An Immense Promise to Fight against Cancer. J. Drug Deliv. 2017, 2017, 1–24. [Google Scholar] [CrossRef]
- Das, S.; Ng, W.K.; Tan, R.B.H. Sucrose ester stabilized solid lipid nanoparticles and nanostructured lipid carriers: I. Effect of formulation variables on the physicochemical properties, drug release and stability of clotrimazole-loaded nanoparticles. Nanotechnology 2014, 25, 105101. [Google Scholar] [CrossRef] [PubMed]
- Eleraky, N.E.; Omar, M.M.; Mahmoud, H.A.; Abou-Taleb, H.A. Nanostructured Lipid Carriers to Mediate Brain Delivery of Temazepam: Design and In Vivo Study. Pharmaceutics 2020, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Paarakh, M.P.; Jose, P.A.; Setty, C.M.; Peter, G.V. Release Kinetics—Cconcepts and Applications. IJPRT 2018, 8, 12. [Google Scholar]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef]
- Diniz, D.M.; Franze, S.; Homberg, J.R. Crossing the Blood-Brain-Barrier: A bifunctional liposome for BDNF gene delivery—A Pilot Study. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Günday Türeli, N. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef]
- Zensi, A.; Begley, D.; Pontikis, C.; Legros, C.; Mihoreanu, L.; Büchel, C.; Kreuter, J. Human serum albumin nanoparticles modified with apolipoprotein A-I cross the blood-brain barrier and enter the rodent brain. J. Drug Target. 2010, 18, 842–848. [Google Scholar] [CrossRef]
- Tosi, G.; Vilella, A.; Veratti, P.; Belletti, D.; Pederzoli, F.; Ruozi, B.; Vandelli, M.A.; Zoli, M.; Forni, F. Exploiting Bacterial Pathways for BBB Crossing with PLGA Nanoparticles Modified with a Mutated Form of Diphtheria Toxin (CRM197): In Vivo Experiments. Mol. Pharm. 2015, 12, 3672–3684. [Google Scholar] [CrossRef]
- Gu, J.; Al-Bayati, K.; Ho, E.A. Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Deliv. Transl. Res. 2017, 7, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, A.; van der Putten, L.; Queiroz, J.; Pinheiro, M.; Reis, S. Transferrin-functionalized lipid nanoparticles for curcumin brain delivery. J. Biotechnol. 2021, 331, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Balasubramanian, S.; Chavan, R.B.; Thipparaboina, R.; Naidu, V.G.M.; Shastri, N.R. Hepatoprotective Cocrystals and Salts of Riluzole: Prediction, Synthesis, Solid State Characterization, and Evaluation. Cryst. Growth Des. 2018, 18, 1047–1061. [Google Scholar] [CrossRef]
- Saitoh, Y.; Takahashi, Y. Riluzole for the treatment of amyotrophic lateral sclerosis. Neurodegener. Dis. Manag. 2020, 10, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, F.; Yanez Arteta, M.; Lerche, M.; Porcar, L.; Lang, C.; Bragg, R.A.; Elmore, C.S.; Krishnamurthy, V.R.; Russell, R.A.; Darwish, T.; et al. Apolipoprotein E Binding Drives Structural and Compositional Rearrangement of mRNA-Containing Lipid Nanoparticles. ACS Nano 2021, 15, 6709–6722. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.; Dimartino, S.; Lee, S.J.; Goh, K.K.; Wen, J.; Oey, I.; Ko, S.; Kwak, H.-S. Interfacial structures of whey protein isolate (WPI) and lactoferrin on hydrophobic surfaces in a model system monitored by quartz crystal microbalance with dissipation (QCM-D) and their formation on nanoemulsions. Food Hydrocoll. 2016, 56, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Baker, H.M.; Baker, E.N. A structural perspective on lactoferrin function. Biochem. Cell Biol. 2012, 90, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Chapple, D.S.; Mason, D.J.; Joannou, C.L.; Odell, E.W.; Gant, V.; Evans, R.W. Structure-Function Relationship of Antibacterial Synthetic Peptides Homologous to a Helical Surface Region on Human Lactoferrin against Escherichia coli Serotype O. Infect. Immun. 1998, 66, 2434–2440. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.P.A.; Talman, V.; Torrieri, G.; Liu, D.; Marques, G.; Moslova, K.; Liu, Z.; Pinto, J.; Hirvonen, J.; Ruskoaho, H.; et al. Dual-Drug Delivery Using Dextran-Functionalized Nanoparticles Targeting Cardiac Fibroblasts for Cellular Reprogramming. Adv. Funct. Mater. 2018, 28, 1705134. [Google Scholar] [CrossRef]
- Shah, R.M.; Eldridge, D.S.; Palombo, E.A.; Harding, I.H. Encapsulation of clotrimazole into solid lipid nanoparticles by microwave-assisted microemulsion technique. Appl. Mater. Today 2016, 5, 118–127. [Google Scholar] [CrossRef]
- Agarwal, S.; Murthy, R.S.R.; Harikumar, S.L.; Garg, R. Quality by Design Approach for Development and Characterisation of Solid Lipid Nanoparticles of Quetiapine Fumarate. Curr. Comput. Drug Des. 2020, 16, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-Y.J.; Chen, W.-J.; Lee, W.-Y.; Lo, S.-T.; Lee, T.-W.; Lo, J.-M. In Vitro and in Vivo Evaluation of Lactoferrin-Conjugated Liposomes as a Novel Carrier to Improve the Brain Delivery. Int. J. Mol. Sci. 2013, 14, 2862–2874. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef] [Green Version]
- Vitorino, C.; Silva, S.; Gouveia, F.; Bicker, J.; Falcão, A.; Fortuna, A. QbD-driven development of intranasal lipid nanoparticles for depression treatment. Eur. J. Pharm. Biopharm. 2020, 153, 106–120. [Google Scholar] [CrossRef]
- Kumbhar, D.D.; Pokharkar, V.B. Engineering of a nanostructured lipid carrier for the poorly water-soluble drug, bicalutamide: Physicochemical investigations. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 416, 32–42. [Google Scholar] [CrossRef]
- Sathyanarayanmoorthi, V.; Karunathan, R.; Kannappan, V. Molecular Modeling and Spectroscopic Studies of Benzothiazole. J. Chem. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, S.; Tang, P.; Yan, J.; Xu, K.; Li, H. Characterization and evaluation of synthetic riluzole with β-cyclodextrin and 2,6-di-O-methyl-β-cyclodextrin inclusion complexes. Carbohydr. Polym. 2015, 129, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.L.; Lima, S.; Vieira, A.C.C.; Ferreira, D.; Sarmento, B.; Reis, S. Overcoming clofazimine intrinsic toxicity: Statistical modelling and characterization of solid lipid nanoparticles. J. R. Soc. Interface 2018, 15, 20170932. [Google Scholar] [CrossRef]
- Doktorovova, S.; Araújo, J.; García, M.L.; Rakovský, E.; Souto, E.B. Formulating fluticasone propionate in novel PEG-containing nanostructured lipid carriers (PEG-NLC). Colloids Surf. B Biointerfaces 2010, 75, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.I.; Silva, A.C.; Catita, J.; Cerqueira, F.; Gabriel, C.; Lopes, C.M. Miconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: Improving antifungal activity. Colloids Surf. B Biointerfaces 2013, 111, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Ramasamy, T.; Truong, D.H.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Preparation and Characterization of Fenofibrate-Loaded Nanostructured Lipid Carriers for Oral Bioavailability Enhancement. AAPS PharmSciTech 2014, 15, 1509–1515. [Google Scholar] [CrossRef] [Green Version]
- Bunjes, H.; Koch, M.H.J.; Westesen, K. Effect of Particle Size on Colloidal Solid Triglycerides. Langmuir 2000, 16, 5234–5241. [Google Scholar] [CrossRef]
- Siahdasht, F.N.; Farhadian, N.; Karimi, M.; Hafizi, L. Enhanced delivery of melatonin loaded nanostructured lipid carriers during in vitro fertilization: NLC formulation, optimization and IVF efficacy. RSC Adv. 2020, 10, 9462–9475. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer-Guglielmi, B.; Jansen, R.-P. The Motor Neuron-Like Cell Line NSC-34 and Its Parent Cell Line N18TG2 Have Glycogen that is Degraded Under Cellular Stress. Neurochem. Res. 2021, 46, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Hounoum, B.M.; Vourc’H, P.; Felix, R.; Corcia, P.; Patin, F.; Guéguinou, M.; Potier-Cartereau, M.; Vandier, C.; Raoul, C.; Andres, C.R.; et al. NSC-34 Motor Neuron-Like Cells Are Unsuitable as Experimental Model for Glutamate-Mediated Excitotoxicity. Front. Cell. Neurosci. 2016, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Weksler, B.; A Romero, I.; Couraud, P.-O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef] [Green Version]







| Excipient | NLC Placebo | NLC Riluzole 1 |
|---|---|---|
| Precirol® ATO5 | 5.250 | 5.250 |
| Miglyol® 812 | 2.250 | 2.250 |
| Stearic acid | 0.125 | 0.125 |
| Riluzole | — | 0.100 |
| Tween® 80 | 2.000 | 2.000 |
| Kolliphor® P 188 micro | 1.000 | 1.000 |
| Ultra-purified water | 89.000 | 88.900 |
| Mean Diameter (nm) 1 | Polydispersity Index 1 | Zeta Potential (mV) 1 | Encapsulation Efficiency (%) 2 | |
|---|---|---|---|---|
| NLC Placebo | 180.3 ± 6.3 a | 0.216 ± 0.023 b | 16.97 ± 4.98 b | — |
| NLC Riluzole | 208.5 ± 5.0 a,b | 0.154 ± 0.023 a | 20.67 ± 2.11 b | 98.70 ± 0.96 a |
| Functionalized NLC | 221.9 ± 18.1 b | 0.219 ± 0.027 b | −16.41 ± 0.31 a | 94.21 ± 4.35 a |
| Kinetic Model | Parameters | NLC Riluzole | Functionalized NLC |
|---|---|---|---|
| Zero-order | 0.0254 | 1.2734 | |
| R2 | 0.9681 | 0.9689 | |
| 0.9617 | 0.9626 | ||
| Higuchi | 0.7353 | 1.9151 | |
| R2 | 0.9945 | 0.9951 | |
| 0.9933 | 0.9941 | ||
| Korsmeyer–Peppas | 0.4999 | 1.2734 | |
| R2 | 0.9950 | 0.9957 | |
| 0.9925 | 0.9935 | ||
| n | 0.5548 | 0.5579 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, M.I.; Lopes, C.M.; Gonçalves, H.; Catita, J.; Silva, A.M.; Rodrigues, F.; Amaral, M.H.; Costa, P.C. Formulation, Characterization, and Cytotoxicity Evaluation of Lactoferrin Functionalized Lipid Nanoparticles for Riluzole Delivery to the Brain. Pharmaceutics 2022, 14, 185. https://doi.org/10.3390/pharmaceutics14010185
Teixeira MI, Lopes CM, Gonçalves H, Catita J, Silva AM, Rodrigues F, Amaral MH, Costa PC. Formulation, Characterization, and Cytotoxicity Evaluation of Lactoferrin Functionalized Lipid Nanoparticles for Riluzole Delivery to the Brain. Pharmaceutics. 2022; 14(1):185. https://doi.org/10.3390/pharmaceutics14010185
Chicago/Turabian StyleTeixeira, Maria Inês, Carla Martins Lopes, Hugo Gonçalves, José Catita, Ana Margarida Silva, Francisca Rodrigues, Maria Helena Amaral, and Paulo C. Costa. 2022. "Formulation, Characterization, and Cytotoxicity Evaluation of Lactoferrin Functionalized Lipid Nanoparticles for Riluzole Delivery to the Brain" Pharmaceutics 14, no. 1: 185. https://doi.org/10.3390/pharmaceutics14010185