Superparamagnetic Iron Oxide Nanoparticles Decorated Mesoporous Silica Nanosystem for Combined Antibiofilm Therapy
Abstract
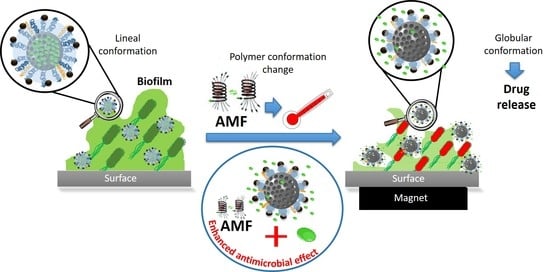
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Synthesis of Materials
2.2.1. Synthesis of Magnetic Nanoparticles (Fe3O4 NPs)
2.2.2. Synthesis of Mesoporous Silica Nanoparticles (MSNs)
2.2.3. Grafting of Polyethyleneglycol to MSNs (MSNf-PEG)
2.2.4. Polymerization of N-Isopropylacrylamide (MSNf-PEG-PNIPAM)
2.2.5. Grafting of Fe3O4 NPs to MSNf-PEG-PNIPAM (mMSNf-PEG-PNIPAM)
2.3. Levofloxacin Loading and Triggered Release
2.3.1. Loading of Levofloxacin (mMSNf-PEG-PNIPAM-L)
2.3.2. In Vial Triggered Levofloxacin Release Assays
2.4. Microbiological Assays
2.4.1. Bacterial Culture
2.4.2. Biofilm Growth
2.4.3. Biofilm Viability Assay
3. Results and Discussion
3.1. Synthesis and Physicochemical Characterization of the Nanosystem
3.2. In Vial Triggered Levofloxacin Release Assays
3.2.1. Temperature as Release Trigger
3.2.2. Magnetic Field as Release Trigger
3.3. Microbiological Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Taubes, G. The bacteria fight back. Science 2008, 321, 356–361. [Google Scholar] [CrossRef]
- World Health Organization. The Top 10 Causes of Death. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death” (accessed on 30 December 2021).
- Davey, M.E.; O’toole, G.A. Microbial Biofilms: From Ecology to Molecular Genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef] [Green Version]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Stewart, P.S. Antimicrobial Tolerance in Biofilms. Microbiol. Spectr. 2015, 3, eLocator: 3.3.07. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durmus, N.G.; Webster, T.J. Eradicating Antibiotic-Resistant Biofilms with Silver-Conjugated Superparamagnetic Iron Oxide Nanoparticles. Adv. Healthc. Mater. 2013, 2, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Borel, N.; Sauer-Durand, A.M.; Hartel, M.; Kuratli, J.; Vaupel, P.; Scherr, N.; Pluschke, G. wIRA: Hyperthermia as a treatment option for intracellular bacteria, with special focus on Chlamydiae and Mycobacteria. Int. J. Hyperth. 2020, 37, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Pijls, B.G.; Sanders, I.M.J.G.; Kuijper, E.J.; Nelissen, R.G.H.H. Synergy between induction heating, antibiotics, and N-acetylcysteine eradicates Staphylococcus aureus from biofilm. Int. J. Hyperth. 2020, 37, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Vallet-Regí, M.; González, B.; Izquierdo-Barba, I. Nanomaterials as promising alternative in the infection treatment. Int. J. Mol. Sci. 2019, 20, 3806. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Bassegoda, A.; Ivanova, K.; Ramon, E.; Tzanov, T. Strategies to prevent the occurrence of resistance against antibiotics by using advanced materials. Appl. Microbiol. Biotechnol. 2018, 102, 2075–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet-Regí, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Castillo, R.R.; Lozano, D.; González, B.; Manzano, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: An update. Expert Opin. Drug Deliv. 2019, 16, 415–439. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous silica nanoparticles for drug delivery: Current insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez, E.; González, B.; Lozano, D.; Doadrio, A.L.; Colilla, M.; Izquierdo-Barba, I. Nanoantibiotics Based in Mesoporous Silica Nanoparticles: New Formulations for Bacterial Infection Treatment. Pharmaceutics 2021, 13, 2033. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.J.; Gisbert-Garzarán, M.; Mediero, A.; Carias-Cálix, R.A.; Jiménez-Jiménez, C.; Esteban, J.; Vallet-Regí, M. Arabic gum plus colistin coated moxifloxacin-loaded nanoparticles for the treatment of bone infection caused by Escherichia coli. Acta Biomater. 2021, 137, 218–237. [Google Scholar] [CrossRef]
- Álvarez, E.; Estévez, M.; Jiménez-Jiménez, C.; Colilla, M.; Izquierdo-Barba, I.; González, B.; Vallet-Regí, M. A versatile multicomponent mesoporous silica nanosystem with dual antimicrobial and osteogenic effects. Acta Biomater. 2021, 136, 570–581. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Gun’ko, Y.K.; Vallet-Regí, M. Mesoporous silica materials as drug delivery: “The nightmare” of bacterial infection. Pharmaceutics 2018, 10, 279. [Google Scholar] [CrossRef] [Green Version]
- Bernardos, A.; Piacenza, E.; Sancenón, F.; Hamidi, M.; Maleki, A.; Turner, R.J.; Martínez-Máñez, R. Mesoporous Silica-Based Materials with Bactericidal Properties. Small 2019, 15, e1900669. [Google Scholar] [CrossRef] [PubMed]
- Colilla, M.; Vallet-Regí, M. Targeted stimuli-responsive mesoporous silica nanoparticles for bacterial infection treatment. Int. J. Mol. Sci. 2020, 21, 8605. [Google Scholar] [CrossRef]
- Yu, E.; Galiana, I.; Martínez-Máñez, R.; Stroeve, P.; Marcos, M.D.; Aznar, E.; Sancenón, F.; Murguía, J.R.; Amorós, P. Poly(N-isopropylacrylamide)-gated Fe3O4/SiO2 core shell nanoparticles with expanded mesoporous structures for the temperature triggered release of lysozyme. Colloids Surf. B Biointerfaces 2015, 135, 652–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Deng, T.; Lin, F.-C.; Zhang, B.; Zink, J.I. Supramolecular Assemblies of Heterogeneous Mesoporous Silica Nanoparticles to Co-deliver Antimicrobial Peptides and Antibiotics for Synergistic Eradication of Pathogenic Biofilms. ACS Nano 2020, 14, 5926–5937. [Google Scholar] [CrossRef] [PubMed]
- García, A.; González, B.; Harvey, C.; Izquierdo-Barba, I.; Vallet-Regí, M. Effective reduction of biofilm through photothermal therapy by gold core@ shell based mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2021, 328, 111489. [Google Scholar] [CrossRef]
- Chmielewski, R.A.N.; Frank, J.F. A predictive model for heat inactivation of Listeria monocytogenes biofilm on buna-N rubber. LWT -Food Sci. Technol. 2006, 39, 11–19. [Google Scholar] [CrossRef]
- O’Toole, A.; Ricker, E.B.; Nuxoll, E. Thermal mitigation of Pseudomonas aeruginosa biofilms. Biofouling 2015, 31, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Salgueiriño-Maceira, V.; Correa-Duarte, M.A.; Farle, M.; López-Quintela, A.; Sieradzki, K.; Diaz, R. Bifunctional gold-coated magnetic silica spheres. Chem. Mater. 2006, 18, 2701–2706. [Google Scholar] [CrossRef]
- Huang, W.C.; Tsai, P.J.; Chen, Y.C. Multifunctional Fe3O4@Au nanoeggs as photothermal agents for selective killing of nosocomial and antibiotic-resistant bacteria. Small 2009, 5, 51–56. [Google Scholar] [CrossRef]
- Wahlen, L.K.; Parker, A.; Walker, D.; Pasmore, M.; Sturman, P. Predictive modeling for hot water inactivation of planktonic and biofilm-associated Sphingomonas parapaucimobilis to support hot water sanitization programs. Biofouling 2016, 32, 751–761. [Google Scholar] [CrossRef]
- Zharov, V.P.; Mercer, K.E.; Galitovskaya, E.N.; Smeltzer, M.S. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys. J. 2006, 90, 619–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Tian, Y.; Cui, Y.; Liu, W.; Ma, W.; Jiang, X. Small molecule-capped gold nanoparticles as potent antibacterial agents that target gram-negative bacteria. J. Am. Chem. Soc. 2010, 132, 12349–12356. [Google Scholar] [CrossRef]
- Huang, W.C.; Tsai, P.J.; Chen, Y.C. Functional gold nanoparticles as photothermal agents for selective-killing of pathogenic bacteria. Nanomedicine 2007, 2, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.S.; Stone, J.W.; Gole, A.; Murphy, C.J.; Sabo-Attwood, T.L. Targeted photothermal lysis of the pathogenic bacteria, Pseudomonas aeruginosa, with gold nanorods. Nano Lett. 2008, 8, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Shiotani, A.; Mori, T.; Niidome, T.; Niidome, Y.; Katayama, Y. Stable incorporation of gold nanorods into N-isopropylacrylamide hydrogels and their rapid shrinkage induced by near-infrared laser irradiation. Langmuir 2007, 23, 4012–4018. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef]
- Ko, S.; Jang, J. A highly efficient palladium nanocatalyst anchored on a magnetically functionalized polymer-nanotube support. Angew. Chem. Int. Ed. 2006, 45, 7564–7567. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Duong, H.T.T.; Selvanayagam, R.; Boyer, C.; Barraud, N. Iron oxide nanoparticle-mediated hyperthermia stimulates dispersal in bacterial biofilms and enhances antibiotic efficacy. Sci. Rep. 2015, 5, 18385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlovsky, L.; Sturtevant, R.A.; Younger, J.G.; Solomon, M.J. Effects of temperature on the morphological, polymeric, and mechanical properties of Staphylococcus epidermidis bacterial biofilms. Langmuir 2015, 31, 2036–2042. [Google Scholar] [CrossRef] [Green Version]
- Richardson, I.P.; Sturtevant, R.; Heung, M.; Solomon, M.J.; Younger, J.G.; VanEpps, J.S. Hemodialysis catheter heat transfer for biofilm prevention and treatment. ASAIO J. 2016, 62, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Toledo, L.d.A.S.; Rosseto, H.C.; Bruschi, M.L. Iron oxide magnetic nanoparticles as antimicrobials for therapeutics. Pharm. Dev. Technol. 2018, 23, 316–323. [Google Scholar] [CrossRef]
- Xu, C.; Akakuru, O.U.; Zheng, J.; Wu, A. Applications of iron oxide-based magnetic nanoparticles in the diagnosis and treatment of bacterial infections. Front. Bioeng. Biotechnol. 2019, 7, 141. [Google Scholar] [CrossRef]
- Kim, M.H.; Yamayoshi, I.; Mathew, S.; Lin, H.; Nayfach, J.; Simon, S.I. Magnetic nanoparticle targeted hyperthermia of cutaneous Staphylococcus aureus infection. Ann. Biomed. Eng. 2013, 41, 598–609. [Google Scholar] [CrossRef] [Green Version]
- Ricker, E.B.; Nuxoll, E. Synergistic effects of heat and antibiotics on Pseudomonas aeruginosa biofilms. Biofouling 2017, 33, 855–866. [Google Scholar] [CrossRef]
- Sturtevant, R.A.; Sharma, P.; Pavlovsky, L.; Stewart, E.J.; Solomon, M.J.; Younger, J.G. Thermal augmentation of vancomycin against staphylococcal biofilms. Shock 2015, 44, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chopra, R.; Shaikh, S.; Chatzinoff, Y.; Munaweera, I.; Cheng, B.; Daly, S.M.; Xi, Y.; Bing, C.; Burns, D.; Greenberg, D.E. Employing high-frequency alternating magnetic fields for the non-invasive treatment of prosthetic joint infections. Sci. Rep. 2017, 7, 7520. [Google Scholar] [CrossRef] [Green Version]
- Gazel, D.; Yilmaz, M. Are infectious diseases and microbiology new fields for thermal therapy research? Int. J. Hyperth. 2018, 34, 918–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falk, M.H.; Issels, R.D. Hyperthermia in oncology. Int. J. Hyperth. 2001, 17, 1–18. [Google Scholar] [CrossRef]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colilla, M.; Balas, F.; Manzano, M.; Vallet-Regí, M. Novel method to enlarge the surface area of SBA-15. Chem. Mater. 2007, 19, 3099–3101. [Google Scholar] [CrossRef]
- Johannsen, M.; Thiesen, B.; Wust, P.; Jordan, A. Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperth. 2010, 26, 790–795. [Google Scholar] [CrossRef]
- Rodrigues, D.; Bañobre-López, M.; Espiña, B.; Rivas, J.; Azeredo, J. Effect of magnetic hyperthermia on the structure of biofilm and cellular viability of a food spoilage bacterium. Biofouling 2013, 29, 1225–1232. [Google Scholar] [CrossRef]
- Park, H.; Park, H.J.; Kim, J.A.; Lee, S.H.; Kim, J.H.; Yoon, J.; Park, T.H. Inactivation of Pseudomonas aeruginosa PA01 biofilms by hyperthermia using superparamagnetic nanoparticles. J. Microbiol. Methods 2011, 84, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Barick, K.C.; Bahadur, D. Inactivation of bacterial pathogens under magnetic hyperthermia using Fe3O4-ZnO nanocomposite. Powder Technol. 2015, 269, 513–519. [Google Scholar] [CrossRef]
- Marchianò, V.; Salvador, M.; Moyano, A.; Gutiérrez, G.; Matos, M.; Yáñez-Vilar, S.; Piñeiro, Y.; Rivas, J.; Martínez-García, J.C.; Peddis, D.; et al. Electrodecoration and characterization of superparamagnetic iron oxide nanoparticles with bioactive synergistic nanocopper: Magnetic hyperthermia-induced ionic release for anti-biofilm action. Antibiotics 2021, 10, 119. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Röder, M. Effects of size distribution on hysteresis losses of magnetic nanoparticles for hyperthermia. J. Phys. Condens. Matter 2008, 20, 385214. [Google Scholar] [CrossRef]
- Périgo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and advances in magnetic hyperthermia. Appl. Phys. Rev. 2015, 2, 041302. [Google Scholar] [CrossRef] [Green Version]
- Ovejero, J.G.; Armenia, I.; Serantes, D.; Veintemillas-Verdaguer, S.; Zeballos, N.; López-Gallego, F.; Grüttner, C.; Fuente, J.M.; de la Fuente, J.M.; del Puerto Morales, M.; et al. Selective Magnetic Nanoheating: Combining Iron Oxide Nanoparticles for Multi-Hot-Spot Induction and Sequential Regulation. Nano Lett. 2021, 21, 7213–7220. [Google Scholar] [CrossRef]
- Guisasola, E.; Baeza, A.; Talelli, M.; Arcos, D.; Moros, M.; De La Fuente, J.M.; Vallet-Regí, M. Magnetic-responsive release controlled by hot spot effect. Langmuir 2015, 31, 12777–12782. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, H.; Salas, G.; Arias-Gonzalez, J.R. Heat generation in single magnetic nanoparticles under near-infrared irradiation. ACS Appl. Mater. Interfaces 2020, 11, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Guisasola, E.; Ruiz-Hernández, E.; Vallet-Regí, M. Magnetically triggered multidrug release by hybrid mesoporous silica nanoparticles. Chem. Mater. 2012, 24, 517–524. [Google Scholar] [CrossRef]
- Zhang, C.; Du, C.; Liao, J.-Y.; Gu, Y.; Gong, Y.; Pei, J.; Gu, H.; Yin, D.; Gao, L.; Pan, Y. Synthesis of magnetite hybrid nanocomplexes to eliminate bacteria and enhance biofilm disruption. Biomater. Sci. 2019, 7, 2833–2840. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Li, P.; Wang, L.; Zhou, J.; Zhang, G.; Li, X.; Hu, B.; Xing, X. Microenvironment-responsive magnetic nanocomposites based on silver nanoparticles/gentamicin for enhanced biofilm disruption by magnetic field. ACS Appl. Mater. Interfaces 2018, 10, 34905–34915. [Google Scholar] [CrossRef]
- Tan, M.; Reyes-Ortega, F.; Schneider-Futschik, E.K. Magnetic nanoparticle-based drug delivery approaches for preventing and treating biofilms in cystic fibrosis. Magnetochemistry 2020, 6, 72. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Felix, R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Hiergeist, R.; Andrä, W.; Buske, N.; Hergt, R.; Hilger, I.; Richter, U.; Kaiser, W. Application of magnetite ferrofluids for hyperthermia. J. Magn. Magn. Mater. 1999, 201, 420–422. [Google Scholar] [CrossRef]
- Hergt, R.; Hiergeist, R.; Hilger, I.; Kaiser, W.A.; Lapatnikov, Y.; Margel, S.; Richter, U. Maghemite nanoparticles with very high AC-losses for application in RF-magnetic hyperthermia. J. Magn. Magn. Mater. 2004, 270, 345–357. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Namvar, F.; Nadi, B.; Rahman, M.Z.; Amin, J. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [Green Version]
- Barani, M.; Zeeshan, M.; Kalantar-Neyestanaki, D.; Farooq, M.A.; Rahdar, A.; Jha, N.K.; Sargazi, S.; Kumar Gupta, P.; Thakur, V.K. Nanomaterials in the Management of Gram-Negative Bacterial Infections. Nanomaterials 2021, 11, 2535. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Z.; Xiang, H.; Xu, X.; Zou, J.; Lu, C. Biocompatible Superparamagnetic Europium-Doped Iron Oxide Nanoparticle Clusters as Multifunctional Nanoprobes for Multimodal In Vivo Imaging. ACS Appl. Mater. Interfaces 2021, 13, 33850–33861. [Google Scholar] [CrossRef]
- Elahi, N.; Rizwan, M. Progress and prospects of magnetic iron oxide nanoparticles in biomedical applications: A review. Artif. Organs. 2021, 45, 1272–1299. [Google Scholar] [CrossRef]
- Alumutairi, L.; Yu, B.; Filka, M.; Nayfach, J.; Kim, M.H. Mild magnetic nanoparticle hyperthermia enhances the susceptibility of Staphylococcus aureus biofilm to antibiotics. Int. J. Hyperth. 2020, 37, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, L.; Pan, J.; Zhao, J.; Tang, J.; Jiang, D.; Hu, P.; Jia, W.; Shi, J. Magneto-based synergetic therapy for implant-associated infections via biofilm disruption and innate immunity regulation. Adv. Sci. 2021, 8, 2004010. [Google Scholar] [CrossRef] [PubMed]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 14813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas, G.; Casado, C.; Teran, F.J.; Miranda, R.; Serna, C.J.; Morales, M.P. Controlled synthesis of uniform magnetite nanocrystals with high-quality properties for biomedical applications. J. Mater. Chem. 2012, 22, 21065–21075. [Google Scholar] [CrossRef]
- Gutiérrez, L.; De La Cueva, L.; Moros, M.; Mazarío, E.; De Bernardo, S.; De La Fuente, J.M.; Morales, M.P.; Salas, G. Aggregation effects on the magnetic properties of iron oxide colloids. Nanotechnology 2019, 30, 112001. [Google Scholar] [CrossRef] [Green Version]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Anson Moye, H.; Boning, A.J. A versatile fluorogenic labelling reagent for primary and secondary amines: 9-fluorenylmethyl chloroformate. Anal. Lett. 1979, 12, 25–35. [Google Scholar] [CrossRef]
- Hedges, A.J. Estimating the precision of serial dilutions and viable bacterial counts. Int. J. Food Microbiol. 2002, 76, 207–214. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-Ray Diffraction, 3rd ed.; Addison-Wesley Publishing Company, Inc.: Boston, MA, USA, 1978; ISBN 0201011743. [Google Scholar]
- Guardia, P.; Batlle-Brugal, B.; Roca, A.G.; Iglesias, O.; Morales, M.P.; Serna, C.J.; Labarta, A.; Batlle, X. Surfactant effects in magnetite nanoparticles of controlled size. J. Magn. Magn. Mater. 2007, 316, 756–759. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, Y.; Goodarzi, A.; Swihart, M.T.; Ohulchanskyy, T.Y.; Kaur, N.; Furlani, E.P.; Prasad, P.N. Aqueous ferrofluid of magnetite nanoparticles: Fluorescence labeling and magnetophoretic control. J. Phys. Chem. B 2005, 109, 3879–3885. [Google Scholar] [CrossRef]
- Williams, A.; Ibrahim, I.T. Carbodiimide chemistry: Recent advances. Chem. Rev. 1981, 81, 589–636. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Colilla, M.; Izquierdo-Barba, I.; Sánchez-Salcedo, S.; Fierro, J.L.G.; Hueso, J.L.; Vallet-Regí, M. Synthesis and characterization of zwitterionic SBA-15 nanostructured materials. Chem. Mater. 2010, 22, 6459–6466. [Google Scholar] [CrossRef]
- González, B.; Colilla, M.; Vallet-Regí, M. Time-delayed release of bioencapsulates: A novel controlled delivery concept for bone implant technologies. Chem. Mater. 2008, 20, 4826–4834. [Google Scholar] [CrossRef]
- González, B.; Colilla, M.; Díez, J.; Pedraza, D.; Guembe, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous silica nanoparticles decorated with polycationic dendrimers for infection treatment. Acta Biomater. 2018, 68, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Colilla, M.; Balas, F.; Vallet-Regí, M. Surface electrochemistry of mesoporous silicas as a key factor in the design of tailored delivery devices. Langmuir 2010, 26, 5038–5049. [Google Scholar] [CrossRef]
- Harder, P.; Grunze, M.; Dahint, R.; Whitesides, G.M.; Laibinis, P.E. Molecular conformation in oligo(ethylene glycol)-terminated self-assembled monolayers on gold and silver surfaces determines their ability to resist protein adsorption. J. Phys. Chem. B 1998, 102, 426–436. [Google Scholar] [CrossRef]
- Futscher, M.H.; Philipp, M.; Müller-Buschbaum, P.; Schulte, A. The Role of Backbone Hydration of Poly(N-isopropyl acrylamide) Across the Volume Phase Transition Compared to its Monomer. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, S.J.; Joy, M.; Maity, T.; Chakraborty, J.; Kotnala, R.K.; Ghosh, S. Colloidal properties of water dispersible magnetite nanoparticles by photon correlation spectroscopy. RSC Adv. 2016, 6, 14393–14402. [Google Scholar] [CrossRef]
- Palma, S.I.C.J.; Marciello, M.; Carvalho, A.; Veintemillas-Verdaguer, S.; del Puerto Morales, M.; Roque, A.C.A. Effects of phase transfer ligands on monodisperse iron oxide magnetic nanoparticles. J. Colloid Interface Sci. 2015, 437, 147–155. [Google Scholar] [CrossRef]
- Begin-Colin, S.; Felder-Flesch, D. Magnetic Nanoparticles: From Fabrication to Clinical Applications; Thanh, N.T., Ed.; CRC Press: Boca Raton, FL, USA, 2012; p. 616. [Google Scholar]
- Navarro, R.E.; Aguilera-Márquez, D.; Virués, C.; Inoue, M. Hydrogen bonding between carboxylic acids and amide-based macrocycles in their host-guest complexes. Supramol. Chem. 2008, 20, 737–742. [Google Scholar] [CrossRef]
- Balas, F.; Manzano, M.; Colilla, M.; Vallet-Regí, M. L-Trp adsorption into silica mesoporous materials to promote bone formation. Acta Biomater. 2008, 4, 514–522. [Google Scholar] [CrossRef]
- Spirou, S.V.; Basini, M.; Lascialfari, A.; Sangregorio, C.; Innocenti, C. Magnetic hyperthermia and radiation therapy: Radiobiological principles and current practice. Nanomaterials 2018, 8, 401. [Google Scholar] [CrossRef] [Green Version]
- Polo-Corrales, L.; Rinaldi, C. Monitoring iron oxide nanoparticle surface temperature in an alternating magnetic field using thermoresponsive fluorescent polymers. J. Appl. Phys. 2012, 111, 07B334. [Google Scholar] [CrossRef]
- Subbiahdoss, G.; Sharifi, S.; Grijpma, D.W.; Laurent, S.; Van Der Mei, H.C.; Mahmoudi, M.; Busscher, H.J. Magnetic targeting of surface-modified superparamagnetic iron oxide nanoparticles yields antibacterial efficacy against biofilms of gentamicin-resistant staphylococci. Acta Biomater. 2012, 8, 2047–2055. [Google Scholar] [CrossRef]






| Sample | DH (nm) | ζ-Potential (mV) |
|---|---|---|
| MSN | 160 ± 30 | 22 ± 4 |
| MSNf | 214 ± 13 | 43 ± 1 |
| MSNf-PEG | 221 ± 23 | 34 ± 2 |
| MSNf-PEG-PNIPAM | 255 ± 18 | 9 ± 1 |
| mMSNf-PEG-PNIPAM | 255 ± 20 | −6 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, E.; Estévez, M.; Gallo-Cordova, A.; González, B.; Castillo, R.R.; Morales, M.d.P.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Superparamagnetic Iron Oxide Nanoparticles Decorated Mesoporous Silica Nanosystem for Combined Antibiofilm Therapy. Pharmaceutics 2022, 14, 163. https://doi.org/10.3390/pharmaceutics14010163
Álvarez E, Estévez M, Gallo-Cordova A, González B, Castillo RR, Morales MdP, Colilla M, Izquierdo-Barba I, Vallet-Regí M. Superparamagnetic Iron Oxide Nanoparticles Decorated Mesoporous Silica Nanosystem for Combined Antibiofilm Therapy. Pharmaceutics. 2022; 14(1):163. https://doi.org/10.3390/pharmaceutics14010163
Chicago/Turabian StyleÁlvarez, Elena, Manuel Estévez, Alvaro Gallo-Cordova, Blanca González, Rafael R. Castillo, María del Puerto Morales, Montserrat Colilla, Isabel Izquierdo-Barba, and María Vallet-Regí. 2022. "Superparamagnetic Iron Oxide Nanoparticles Decorated Mesoporous Silica Nanosystem for Combined Antibiofilm Therapy" Pharmaceutics 14, no. 1: 163. https://doi.org/10.3390/pharmaceutics14010163