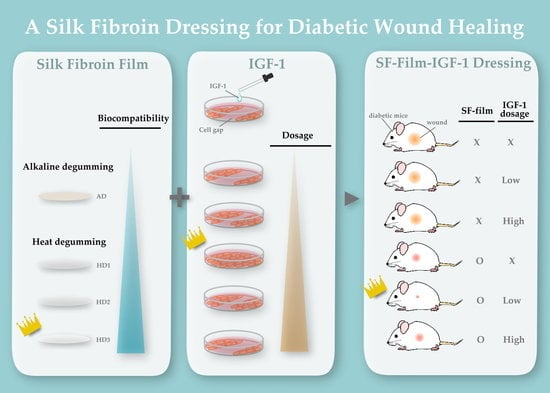
An Insulin-like Growth Factor-1 Conjugated Bombyx mori Silk Fibroin Film for Diabetic Wound Healing: Fabrication, Physicochemical Property Characterization, and Dosage Optimization In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of SF-Films
2.2. Determination of the Purity and Quantity of Silk Fibroin
2.3. Characterization of Physicolchemical Properties of SF-Films
2.4. Determination of Biocompatibility of the SF-Films
2.5. Conjugation of IGF-1 on SF-Films
2.6. Determination of Optimal IGF-1 Dosage for Cell Migration
2.7. Determination of IGF-1 Dosage Effect on Wound Healing in Diabetic Mice
2.8. Histological and Immunohistochemical Analyses
2.9. Statistical Analyses
3. Results
3.1. Manufacturing Procedure of SF-Films
3.2. Effect of Different IGF-1 Dosages on BALB/3T3 Cells Grown in Hyperglycemic Medium
3.3. Effects of SF-Film Loaded with IGF-1 (SF-Film–IGF-1) on Wound Healing of BALB/3T3 Monolayers Grown in Hyperglycemic Medium
3.4. In Vivo Dosage Studies of IGF-1 Loaded onto SF-Films
3.5. Histology of Regenerated Tissue in SF-Film-Treated Diabetic Wounds
4. Discussion
4.1. Manufacturing Procedure of Silk Fibroin (SF)-Film
4.2. Effects of Different Dosage and Release forms of IGF-1
4.3. Effects of Different IGF-1 Dosage on Wound Healing
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Yach, D.; Stuckler, D.; Brownell, K.D. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat. Med. 2006, 12, 62–67. [Google Scholar] [CrossRef]
- Lamers, M.L.; Almeida, M.E.; Vicente-Manzanares, M.; Horwitz, A.F.; Santos, M.F. High glucose-mediated oxidative stress impairs cell migration. PLoS ONE 2011, 6, e22865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randeria, P.S.; Seeger, M.A.; Wang, X.Q.; Wilson, H.; Shipp, D.; Mirkin, C.A.; Paller, A.S. siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown. Proc. Natl. Acad. Sci. USA 2015, 112, 5573–5578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everett, E.; Mathioudakis, N. Update on management of diabetic foot ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wang, S.; He, Y.; Xu, L.; Zhang, S.; Tang, Z. Reasonable glycemic control would help wound healing during the treatment of diabetic foot ulcers. Diabetes Ther. 2019, 10, 95–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ram, M.; Singh, V.; Kumawat, S.; Kant, V.; Tandan, S.K.; Kumar, D. Bilirubin modulated cytokines, growth factors and angiogenesis to improve cutaneous wound healing process in diabetic rats. Int. Immunopharmacol. 2016, 30, 137–149. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.P.; Kirsner, R.S. Angiogenesis in wound repair: Angiogenic growth factors and the extracellular matrix. Microsc. Res. Tech. 2003, 60, 107–114. [Google Scholar] [CrossRef]
- Soneja, A.; Drews, M.; Malinski, T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol. Rep. 2005, 57, 108–119. [Google Scholar]
- Blakytny, R.; Jude, E.B.; Gibson, J.M.; Boulton, A.J.M.; Ferguson, M.W.J. Lack of insulin-like growth factor 1 (IGF1) in the basal keratinocyte layer of diabetic skin and diabetic foot ulcers. J. Pathol. 2000, 190, 589–594. [Google Scholar] [CrossRef]
- Wu, Y.C.; Zhu, M.; Robertson, D.M. Novel nuclear localization and potential function of insulin-like growth factor-1 receptor/insulin receptor hybrid in corneal epithelial cells. PLoS ONE 2012, 7, e42483. [Google Scholar] [CrossRef]
- Jung, H.J.; Suh, Y. Regulation of IGF -1 signaling by microRNAs. Front. Genet. 2015, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nagano, T.; Nakamura, M.; Nakata, K.; Yamaguchi, T.; Takase, K.; Okahara, A.; Ikuse, T.; Nishida, T. Effects of substance P and IGF-1 in corneal epithelial barrier function and wound healing in a rat model of neurotrophic keratopathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3810–3815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuard, W.L.; Titone, R.; Robertson, D.M. The IGF/insulin-IGFBP axis in corneal development, wound healing, and disease. Front. Endocrinol. 2020, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Guler, H.P.; Zapf, J.; Schmid, C.; Froesch, E.R. Insulin-like growth factors I and II in healthy man. estimations of half-lives and production rates. Acta Endocrinol. 1989, 121, 753–758. [Google Scholar] [CrossRef]
- Demling, R.H. The role of anabolic hormones for wound healing in catabolic states. J. Burns Wounds 2005, 17, 46–62. [Google Scholar]
- Cao, Y.; Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Li, Y.; Xie, M.-B. Silk fibroin-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Ling, S.; Li, C.; Omenetto, F.G.; Kaplan, D.L. Silkworm silk-based materials and devices generated using bio-nanotechnology. Chem. Soc. Rev. 2018, 47, 6486–6504. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Johnson, M.A.; Martin, D.C. Microstructural characterization of Bombyx mori silk fibers. Macromolecules 1998, 31, 8857–8864. [Google Scholar] [CrossRef]
- Yucel, T.; Lovett, M.L.; Kaplan, D.L. Silk-based biomaterials for sustained drug delivery. J. Control Release 2014, 190, 381–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padol, A.R.; Jayakumar, K.; Shridhar, N.B.; Swamy, H.D.N.; Mohan, K.S.M. Efficacy of the silk protein based biofilms as a novel wound healing agent. Int. J. Toxicol. Appl. Pharm. 2012, 2, 31–36. [Google Scholar]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of silk fibroinuse in wound dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
- Tran, S.H.; Wilson, C.G.; Seib, F.P. A review of the emerging role of silk for the treatment of the eye. Pharm. Res. 2018, 35, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Gil, E.S.; Panilaitis, B.; Bellas, E.; Kaplan, D.L. Functionalized silk biomaterials for wound healing. Adv. Healthc. Mater. 2013, 2, 206–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, Y.H.; Peng, L.H.; Liu, X.; Chen, X.; Xiong, J.; Gao, J.Q. Silk fibroin/gelatin electrospun nanofibrous dressing functionalized with astragaloside IV induces healing and anti-scar effects on burn wound. Int. J. Pharm. 2015, 479, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.D.; Marchant, J.K.; Pindrus, M.A.; Omenetto, F.G.; Kaplan, D.L. Silk film biomaterials for cornea tissue engineering. Biomaterials 2009, 30, 1299–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, E.S.; Mandal, B.B.; Park, S.H.; Marchant, J.K.; Omenetto, F.G.; Kaplan, D.L. Helicoidal multi-lamellar features of RGD-functionalized silk biomaterials for corneal tissue engineering. Biomaterials 2010, 31, 8953–8963. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lawrence, B.D.; Liu, A.; Schwab, I.R.; Oliveira, L.A.; Rosenblatt, M.I. Silk fibroin as a biomaterial substrate for corneal epithelial cell sheet generation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4130–4138. [Google Scholar] [CrossRef] [Green Version]
- Kambe, Y.; Kojima, K.; Tamada, Y.; Tomita, N.; Kameda, T. Silk fibroin sponges with cell growth-promoting activity induced by genetically fused basic fibroblast growth factor. J. Biomed. Mater. Res. A 2016, 104, 82–93. [Google Scholar] [CrossRef]
- Yodmuang, S.; McNamara, S.L.; Nover, A.B.; Mandal, B.B.; Agarwal, M.; Kelly, T.A.; Chao, P.H.; Hung, C.; Kaplan, D.L.; Vunjak-Novakovic, G. Silk microfiber–reinforced silk hydrogel composites for functional cartilage tissue repair. Acta Biomater. 2015, 11, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Min, B.M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef]
- Schneider, A.; Wang, X.Y.; Kaplan, D.L.; Garlick, J.A.; Egles, C. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 2009, 5, 2570–2578. [Google Scholar] [CrossRef] [Green Version]
- Wittmer, C.R.; Claudepierre, T.; Reber, M.; Wiedemann, P.; Garlick, J.A.; Kaplan, D.; Egles, C. Multifunctionalized electrospun silk fibers promote axon regeneration in central nervous system. Adv. Funct. Mater. 2011, 21, 4202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.-J.; Lu, M.-C.; Chang, H.-Y. Sustained release of insulin-like growth factor-1 from Bombyx mori L. silk fibroin delivery for diabetic wound therapy. Int. J. Mol. Sci. 2021, 22, 6267. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Gulrajanii, M.L. Comparative evaluation of the various methods of degumming silk. Indian J. Fibre Text. Res. 1994, 19, 76–83. [Google Scholar]
- Allardyce, B.J.; Rajkhowa, R.; Dilley, R.J.; Atlas, M.D.; Kaur, J.; Wang, X. The impact of degumming conditions on the properties of silk films for biomedical applications. Text. Res. J. 2015, 86, 275–287. [Google Scholar] [CrossRef]
- Lee, J.; Song, D.; Park, Y.H.; Um, I. Effect of residual sericin on the structural characteristics and properties of regenerated silk films. Int. J. Biol. Macromol. 2016, 89. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.K.; Choi, J.; Hasturk, O.; Laubach, I.; Descoteaux, M.L.; Mosurkal, S.; Wang, B.; Zhang, N.; Kaplan, D.L. Silk degumming time controls horseradish peroxidase-catalyzed hydrogel properties. Biomater. Sci. 2020, 8, 4176–4185. [Google Scholar] [CrossRef]
- Aramwit, P.; Damrongsakkul, S.; Kanokpanont, S.; Srichana, T. Properties and antityrosinase activity of sericin from various extraction methods. Biotechnol. Appl. Biochem. 2010, 55, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kunz, R.I.; Brancalhao, R.M.; Ribeiro, L.F.; Natali, M.R. Silkworm sericin: Properties and biomedical applications. Biomed. Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef] [Green Version]
- Jusu, S.M.; Obayemi, J.D.; Salifu, A.A.; Nwazojie, C.C.; Uzonwanne, V.; Odusanya, O.S.; Soboyejo, W.O. Drug-encapsulated blend of PLGA-PEG microspheres: In vitro and in vivo study of the effects of localized/targeted drug delivery on the treatment of triple-negative breast cancer. Sci. Rep. 2020, 10, 14188. [Google Scholar] [CrossRef]
- Dong, A.; Huang, P.; Caughey, W.S. Redox-dependent changes in ß-extended chain and turn structures of cytochrome c in water solution determined by second derivative amide I infrared spectra. Biochemistry 1992, 31, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Gokuldas, M. Influence of organic solvents on the structural and thermal characteristics of silk protein from the web of Orthaga exvinacea Hampson (Lepidoptera: Pyralidae). J. Chem. Biol. 2016, 9, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Freddi, G.; Pessina, G.; Tsukada, M. Swelling and dissolution of silk fibroin (Bombyx mori) in N-methyl morpholine N-oxide. Int. J. Biol. Macromol. 1999, 24, 251–263. [Google Scholar] [CrossRef]
- Ha, S.-W.; Tonelli, A.E.; Hudson, S.M. Structural studies of Bombyx mori silk Fibroin during regeneration from solutions and wet fiber spinning. Biomacromolecules 2005, 6, 1722–1731. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.-L.; Dai, F.-Y.; Zhang, H.-H.; Ni, B.; Zhou, W.; Yang, X.; Wu, Y.-z. Preparation and characterization of silk fibroin as a biomaterial with potential for drug delivery. J. Transl. Med. 2012, 10, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamalh, E.; Zheng, Y.; Zeng, Y. Analysis of the secondary crystalline structure of regenerated Bombyx mori fibroin. Res. Rev. Biosci. 2013, 7, 76–83. [Google Scholar]
- Horan, R.L.; Antle, K.; Collette, A.L.; Wang, Y.; Huang, J.; Moreau, J.E.; Volloch, V.; Kaplan, D.L.; Altman, G.H. In vitro degradation of silk fibroin. Biomaterials 2005, 26, 3385–3393. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Wray, L.S.; Hu, X.; Gallego, J.; Georgakoudi, I.; Omenetto, F.G.; Schmidt, D.; Kaplan, D.L. Effect of processing on silk-based biomaterials: Reproducibility and biocompatibility. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Lewandowska, K.; Michalska, M.; Walczak, M. Characterization of silk fibroin 3D composites modified by collagen. J. Mol. Liq. 2016, 215, 323–327. [Google Scholar] [CrossRef]
- Yazawa, K.; Ishida, K.; Masunaga, H.; Hikima, T.; Numata, K. Influence of water content on the beta-sheet formation, thermal stability, water removal, and mechanical properties of silk materials. Biomacromolecules 2016, 17, 1057–1066. [Google Scholar] [CrossRef]
- Laron, Z. Insulin-like growth factor 1 (IGF-1): A growth hormone. Mol. Path. 2001, 54, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, C.; Mohan, S.; Sjogren, K.; Tivesten, A.; Isgaard, J.; Isaksson, O.; Jansson, J.O.; Svensson, J. The role of liver-derived insulin-like growth factor-I. Endocr. Rev. 2009, 30, 494–535. [Google Scholar] [CrossRef] [Green Version]
- Bright, G.M. Recombinant IGF-I: Past, present and future. Growth Horm. IGF Res. 2016, 28, 62–65. [Google Scholar] [CrossRef]
- Ando, Y.; Jensen, P.J. Epidermal growth factor and insulin-like growth factor I enhance keratinocyte migration. J. Investig. Dermatol. 1993, 100, 633–639. [Google Scholar] [CrossRef] [Green Version]
- Haase, I.; Evans, R.; Pofahl, R.; Watt, F.M. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J. Cell Sci. 2003, 116, 3227–3238. [Google Scholar] [CrossRef] [Green Version]
- Delafontaine, P.; Song, Y.H.; Li, Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 435–444. [Google Scholar] [CrossRef]
- Sadagurski, M.; Yakar, S.; Weingarten, G.; Holzenberger, M.; Rhodes, C.J.; Breitkreutz, D.; Leroith, D.; Wertheimer, E. Insulin-like growth factor 1 receptor signaling regulates skin development and inhibits skin keratinocyte differentiation. Mol. Cell. Biol. 2006, 26, 2675–2687. [Google Scholar] [CrossRef] [Green Version]
- Balaji, S.; LeSaint, M.; Bhattacharya, S.S.; Moles, C.; Dhamija, Y.; Kidd, M.; Le, L.D.; King, A.; Shaaban, A.; Crombleholme, T.M.; et al. Adenoviral-mediated gene transfer of insulin-like growth factor 1 enhances wound healing and induces angiogenesis. J. Surg. Res. 2014, 190, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Emmerson, E.; Campbell, L.; Davies, F.C.; Ross, N.L.; Ashcroft, G.S.; Krust, A.; Chambon, P.; Hardman, M.J. Insulin-like growth factor-1 promotes wound healing in estrogen-deprived mice: New insights into cutaneous IGF-1R/ERalpha cross talk. J. Invest. Dermatol. 2012, 132, 2838–2848. [Google Scholar] [CrossRef] [Green Version]
- Achar, R.A.N.; SilvaI, T.C.; Achar, E.; Martines, R.B.; Machado, J.L.M. Use of insulin-like growth factor in the healing of open wounds in diabetic and non-diabetic rats. Acta Cir. Bras. 2014, 29, 125–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortved, K.F.; Begum, L.; Mohammed, H.O.; Nixon, A.J. Implantation of rAAV5-IGF-I transduced autologous chondrocytes improves cartilage repair in full-thickness defects in the equine model. Mol. Ther. 2015, 23, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, L.; Yang, W.; Cao, Y.; Shi, Y.; Li, X.; Zhang, Q. The effects of different doses of IGF-1 on cartilage and subchondral bone during the repair of full-thickness articular cartilage defects in rabbits. Osteoarthr. Cartil. 2017, 25, 309–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, N.A.; Barrow, R.E.; Herndon, D.N. Combined insulin-like growth factor-1 and growth hormone improves weight loss and wound healing in burned rats. J. Trauma 1996, 41, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Dunaiski, V.; Belford, D.A. Contribution of circulating IGF-I to wound repair in GH-treated rats. Growth Horm. IGF Res. 2002, 12, 381–387. [Google Scholar] [CrossRef]
- Botusan, I.R.; Zheng, X.; Narayanan, S.; Grunler, J.; Sunkari, V.G.; Calissendorff, F.S.; Ansurudeen, I.; Illies, C.; Svensson, J.; Jansson, J.O.; et al. Deficiency of liver-derived insulin-like growth factor-I (IGF-I) does not interfere with the skin wound healing rate. PLoS ONE 2018, 13, e0193084. [Google Scholar] [CrossRef] [PubMed] [Green Version]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.-J.; Lu, M.-C.; Chan, Y.-C.; Huang, Y.-F.; Chang, H.-Y. An Insulin-like Growth Factor-1 Conjugated Bombyx mori Silk Fibroin Film for Diabetic Wound Healing: Fabrication, Physicochemical Property Characterization, and Dosage Optimization In Vitro and In Vivo. Pharmaceutics 2021, 13, 1459. https://doi.org/10.3390/pharmaceutics13091459
Lin M-J, Lu M-C, Chan Y-C, Huang Y-F, Chang H-Y. An Insulin-like Growth Factor-1 Conjugated Bombyx mori Silk Fibroin Film for Diabetic Wound Healing: Fabrication, Physicochemical Property Characterization, and Dosage Optimization In Vitro and In Vivo. Pharmaceutics. 2021; 13(9):1459. https://doi.org/10.3390/pharmaceutics13091459
Chicago/Turabian StyleLin, Meng-Jin, Mei-Chun Lu, Yun-Chen Chan, Yu-Fen Huang, and Hwan-You Chang. 2021. "An Insulin-like Growth Factor-1 Conjugated Bombyx mori Silk Fibroin Film for Diabetic Wound Healing: Fabrication, Physicochemical Property Characterization, and Dosage Optimization In Vitro and In Vivo" Pharmaceutics 13, no. 9: 1459. https://doi.org/10.3390/pharmaceutics13091459