Nanostructured Lipid Carriers for the Formulation of Topical Anti-Inflammatory Nanomedicines Based on Natural Substances
Abstract
:1. Introduction
2. The Skin
3. Inflammatory Process of the Skin
4. Natural Substances with Anti-Inflammatory Activity
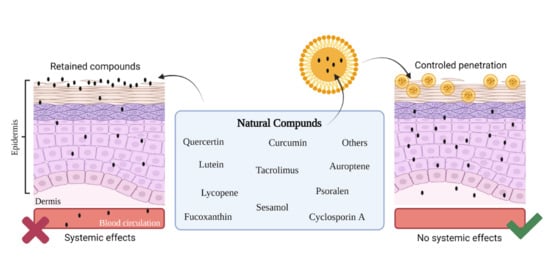
5. Nanostructured Lipid Carriers (NLC)
Constituents and Methods
- Ultrasound: In this method, the particles are formed by ultrasonic waves that generate cavitation in liquids. Thus, when a liquid is subjected to the process of sonication with high intensity, the sound waves propagate in the middle of the liquid, creating alternation of high- and low-pressure sound waves. In the phase of low pressure and high intensity, the waves produce vacuum bubbles, which increase the diameter by absorbing energy. After reaching the high-pressure phase, the bubbles are compressed until they implode [126,127]. By using this method, researchers have to strictly control the conditions to avoid wide particle size distribution, which leads to physical instabilities of the formulation [100,125].
- Microemulsion: This method was first used by Gasco et al. (1997) [96,109,114]. Microemulsions typically contain unsaturated fatty acids, surfactants, co-surfactants, and water. They are mixed at low-speed stirring, which forms an optically transparent mixture at 65–70 °C. The hot microemulsion is then dispersed in cold water (2–3 °C) under gentle agitation resulting in the solidification of nanostructured lipid carriers [100,109,114,121,125].
6. NLC Containing Natural Substances against Skin Inflammation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Martínez-Vitela, C.; Serna-Saldívar, S.O. Topical anti-inflammatory effects of isorhamnetin glycosides isolated from Opuntia ficus-indica. BioMed Res. Int. 2015, 2015, 847320. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.S.; Kim, S.Y.; Kong, B.J.; Kim, K.J.; Noh, G.Y.; Im, N.R.; Lim, J.W.; Ha, J.H.; Kim, J.; Park, S.N. Cell penetrating peptide conjugated liposomes as transdermal delivery system of Polygonum aviculare L. extract. Int. J. Pharm. 2015, 483, 26–37. [Google Scholar] [CrossRef]
- Fuchs, E. Scratching the surface of skin development. Nature 2007, 445, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Yagi, M.; Yonei, Y. Glycative stress and anti-aging: 7. Glycative stress and skin aging. Glycative Stress Res. 2018, 5, 50–54. [Google Scholar]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 16082. [Google Scholar] [CrossRef]
- Torres, F.; das Graças, M.; Melo, M.; Tosti, A. Management of contact dermatitis due to nickel allergy: An update. Clin. Cosmet. Investig. Dermatol. 2009, 2, 39–48. [Google Scholar]
- Chen, H.; Liu, G.; Huang, N.; Li, W.; Dong, X.; Zhu, R. Incidence of allergic contact sensitization in central Chinese subjects with chronic urticaria. An. Bras. Dermatol. 2016, 91, 168–172. [Google Scholar] [CrossRef] [Green Version]
- Bjerre, R.D.; Bandier, J.; Skov, L.; Engstrand, L.; Johansen, J.D. The role of the skin microbiome in atopic dermatitis: A systematic review. Br. J. Dermatol. 2017, 177, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B. What is new in the pathophysiology of acne, an overview. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 8–12. [Google Scholar] [CrossRef]
- Richmond, J.M.; Harris, J.E. Immunology and skin in health and disease. Cold Spring Harb. Perspect. Med. 2014, 4, a015339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [Green Version]
- Ingram, S.L.; Diotallevi, M. Reactive oxygen species: Rapid fire inflammation. Biochemist 2017, 39, 30–33. [Google Scholar] [CrossRef]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlazzo, N.; Cirmi, S.; Calapai, G.; Ventura-Spagnolo, E.; Gangemi, S.; Navarra, M. Anti-Inflammatory Activity of Citrus bergamia Derivatives: Where Do We Stand? Molecules 2016, 21, 1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conte, R.; Marturano, V.; Peluso, G.; Calarco, A.; Cerruti, P. Recent Advances in Nanoparticle-Mediated Delivery of Anti-Inflammatory Phytocompounds. Int. J. Mol. Sci. 2017, 18, 709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puglia, C.; Lauro, M.R.; Offerta, A.; Crascì, L.; Micicchè, L.; Panico, A.M.; Bonina, F.; Puglisi, G. Nanostructured Lipid Carriers (NLC) as Vehicles for Topical Administration of Sesamol: In Vitro Percutaneous Absorption Study and Evaluation of Antioxidant Activity. Planta Med. 2017, 83, 398–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daneshmand, S.; Jaafari, M.R.; Movaffagh, J.; Malaekeh-Nikouei, B.; Iranshahi, M.; Seyedian Moghaddam, A.; Tayarani Najaran, Z.; Golmohammadzadeh, S. Preparation, characterization, and optimization of auraptene-loaded solid lipid nanoparticles as a natural anti-inflammatory agent: In vivo and in vitro evaluations. Colloids Surf. B Biointerfaces 2018, 164, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Castro, G.A.; Oliveira, C.A.; Mahecha, G.A.; Ferreira, L.A. Comedolytic effect and reduced skin irritation of a new formulation of all-trans retinoic acid-loaded solid lipid nanoparticles for topical treatment of acne. Arch. Dermatol. Res. 2011, 303, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-Y.; Yang, C.-F.; Li, Q.L.; Tan, Q.; Xi, Y.-W.; Liu, W.-N.; Zhai, G.-X. Development of a quercetin-loaded nanostructured lipid carrier formulation for topical delivery. Int. J. Pharm. 2012, 430, 292–298. [Google Scholar]
- Kakkar, V.; Kaur, I.P.; Kaur, A.P.; Saini, K.; Singh, K.K. Topical delivery of tetrahydrocurcumin lipid nanoparticles effectively inhibits skin inflammation: In vitro and in vivo study. Drug Dev. Ind. Pharm. 2018, 44, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Schäfer-Korting, M.; Mehnert, W.; Korting, H.C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Küchler, S.; Radowski, M.R.; Blaschke, T.; Dathe, M.; Plendl, J.; Haag, R.; Schäfer-Korting, M.; Kramer, K.D. Nanoparticles for skin penetration enhancement—A comparison of a dendritic core-multishell-nanotransporter and solid lipid nanoparticles. Eur. J. Pharm. Biopharm. 2009, 71, 243–250. [Google Scholar] [CrossRef]
- Bikkad, M.L.; Nathani, A.H.; Mandlik, S.K.; Shrotriya, S.N.; Ranpise, N.S. Halobetasol propionate-loaded solid lipid nanoparticles (SLN) for skin targeting by topical delivery. J. Liposome Res. 2014, 24, 113–123. [Google Scholar] [CrossRef]
- Pang, Z.; Han, C. Review on Transdermal Drug Delivery Systems. J. Pharm. Drug Dev. 2014, 2, 402. [Google Scholar]
- Shrotriya, S.; Ranpise, N.; Satpute, P.; Vidhate, B. Skin targeting of curcumin solid lipid nanoparticles-engrossed topical gel for the treatment of pigmentation and irritant contact dermatitis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1471–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wissing, S.A.; Müller, R.H. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity—In vivo study. Eur. J. Pharm. Biopharm. 2003, 56, 67–72. [Google Scholar] [CrossRef]
- Khalid, K.; Tan, X.; Zaid, H.F.M.; Tao, Y.; Chew, C.L.; Chu, D.T.; Lam, M.K.; Ho, Y.C.; Lim, J.W.; Wei, L.C. Advanced in developmental organic and inorganic nanomaterial: A review. Bioengineered 2020, 11, 328–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, R.E. Nanobiotechnology: Inorganic Nanoparticles vs Organic Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Zhao, Z.; Liu, T.; Zhu, S.; Pi, J.; Guo, P.; Qi, D.; Liu, Z.; Li, N. Natural medicine combined with nanobased topical delivery systems: A new strategy to treat psoriasis. Drug Deliv. Transl. Res. 2021, 1–13. [Google Scholar] [CrossRef]
- Szulc-Musioł, B.; Sarecka-Hujar, B. The Use of Micro- and Nanocarriers for Resveratrol Delivery into and across the Skin in Different Skin Diseases—A Literature Review. Pharmaceutics 2021, 13, 451. [Google Scholar] [CrossRef]
- Biswasroy, P.; Pradhan, D.; Kar, B.; Ghosh, G.; Rath, G. Recent Advancement in Topical Nanocarriers for the Treatment of Psoriasis. AAPS PharmSciTech 2021, 22, 164. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Küchler, S.; Herrmann, W.; Panek-Minkin, G.; Blaschke, T.; Zoschke, C.; Kramer, K.D.; Bittl, R.; Schäfer-Korting, M. SLN for topical application in skin diseases—Characterization of drug-carrier and carrier-target interactions. Int. J. Pharm. 2010, 390, 225–233. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair, and nails. Medicine 2009, 37, 223–226. [Google Scholar] [CrossRef]
- Young, C.N.; Koepke, J.I.; Terlecky, L.J.; Forquin, M.S.; Boyd Savoy, L.; Terlecky, S.R. Reactive oxygen species in tumor necrosis factor-alpha-activated primary human keratinocytes: Implications for psoriasis and inflammatory skin disease. J. Investig. Dermatol. 2008, 128, 2606–2614. [Google Scholar] [CrossRef] [Green Version]
- Wickett, R.R.; Visscher, M.O. Structure and function of the epidermal barrier. Am. J. Infect. Control. 2006, 39, 98–110. [Google Scholar] [CrossRef]
- Barry, B.W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Honeywell-Nguyen, P.L.; Gooris, G.S.; Ponec, M. Structure of the skin barrier and its modulation by vesicular formulations. Prog. Lipid Res. 2003, 42, 1–36. [Google Scholar] [CrossRef]
- Cevc, G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev. 2004, 56, 675–711. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yang, M.; Tang, X.; Wang, T.; Yang, D.; Zhai, G.; Liu, J. Lipid nanoparticles loading triptolide for transdermal delivery: Mechanisms of penetration enhancement and transport properties. J. Nanobiotechnology 2018, 16, 68. [Google Scholar] [CrossRef] [Green Version]
- Khurana, S.; Jain, N.K.; Bedi, P.M. Development and characterization of a novel controlled release drug delivery system based on nanostructured lipid carriers’ gel for meloxicam. Life Sci. 2013, 93, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Arda, O.; Göksügür, N.; Tüzün, Y. Basic histological structure and functions of facial skin. Clin. Dermatol. 2014, 32, 3–13. [Google Scholar] [CrossRef]
- Murphree, R.W. Impairments in Skin Integrity. Nurs. Clin. N. Am. 2017, 52, 405–417. [Google Scholar] [CrossRef]
- Gould, J. Superpowered skin. Nature 2018, 563, S84–S85. [Google Scholar] [CrossRef]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Soulika, A.M. The dynamics of the skin’s immune system. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [Green Version]
- Johansson, J.A.; Headon, D.J. Regionalisation of the skin. Semin. Cell Dev. Biol. 2014, 25–26, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Pando, D.; Matos, M.; Gutiérrez, G.; Pazos, C. Formulation of resveratrol entrapped niosomes for topical use. Colloids Surf. B Biointerfaces 2015, 128, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Dawid-Pać, R. Medicinal plants used in treatment of inflammatory skin diseases. Postepy Dermatol. Alergol. 2013, 30, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Stupin, V.; Manturova, N.; Silina, E.; Litvitskiy, P.; Vasin, V.; Artyushkova, E.; Inanov, A.; Gladchenko, M.; Aliev, S. The effect of inflammation on the healing process of acute skin wounds under the treatment of wounds with injections in rats. J. Exp. Pharmacol. 2020, 12, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the Period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.G.; Rahman, M.M.; Ahmed, N.U.; Fakruddin, M.; Islam, S.; Mazumdar, R.M. Antioxidant, antimicrobial, toxicity, and analgesic properties of ethanol extract of Solena amplexicaulis root. Biol. Res. 2014, 47, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory, and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Phromnoi, K.; Yadav, V.R.; Chaturvedi, M.M.; Aggarwal, B.B. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med. 2010, 76, 1044–1063. [Google Scholar] [CrossRef] [Green Version]
- Cordenonsi, L.M.; Santer, A.; Sponchiado, R.M.; Wingert, N.R.; Raffin, R.P.; Schapoval, E.E.S. Amazonia Products in Novel Lipid Nanoparticles for Fucoxanthin Encapsulation. AAPS PharmSciTech 2019, 21, 32. [Google Scholar] [CrossRef]
- Heo, S.-J.; Yoon, W.J.; Kim, K.N.; Ahn, G.N.; Kang, S.M.; Kang, D.H.; Affan, A.; Oh, C.; Jung, W.K.; Jeon, Y.J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef]
- Oh, J.; Kim, J.H.; Park, J.G.; Yi, Y.S.; Park, K.W.; Rho, H.S.; Lee, M.S.; Yoo, J.W.; Kang, S.H.; Hong, Y.D.; et al. Radical scavenging activity-based and AP-1-targeted anti-inflammatory effects of lutein in macrophage-like and skin keratinocytic cells. Mediat. Inflamm. 2013, 2013, 787042. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.W.S.; Leanderson, P.; Lundberg, A.K.; Jonasson, L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 2017, 262, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamidzadeh, K.; Christensen, S.M.; Dalby, E.; Chandrasekaran, P.; Mosser, D.M. Macrophages and the Recovery from Acute and Chronic Inflammation. Annu. Rev. Physiol. 2017, 79, 567–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landrier, J.F.; Tourniaire, F.; Fenni, S.; Desmarchelier, C.; Borel, P. Tomatoes and lycopene: Inflammatory modulator effects. In Lycopene and Tomatoes in Human Nutrition and Health; Rao, A.V., Young, G.L., Rao, L.G., Eds.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Chen, J.; Song, Y.; Zhang, L. Effect of lycopene supplementation on oxidative stress: An exploratory systematic review and meta-analysis of randomized controlled trials. J. Med. Food 2013, 16, 361–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palozza, P.; Parrone, N.; Catalano, A.; Simone, R. Tomato Lycopene, and Inflammatory Cascade: Basic Interactions and Clinical Implications. Curr. Med. Chem. 2010, 17, 2547–2563. [Google Scholar] [CrossRef]
- Chang, Y.C.; Tsai, M.H.; Sheu, W.H.; Hsieh, S.C.; Chiang, A.N. The therapeutic potential and mechanisms of action of quercetin in relation to lipopolysaccharide-induced sepsis in vitro and in vivo. PLoS ONE 2013, 8, e80744. [Google Scholar] [CrossRef] [Green Version]
- Gunawardena, D.; Govindaraghavan, S.; Münch, G. Anti-Inflammatory Properties of Cinnamon Polyphenols and their Monomeric Precursors. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, UK, 2014; pp. 409–425. [Google Scholar]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Quercetin. 2019. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/quercetin#section=Pharmacology (accessed on 13 April 2020).
- Ha, T.K.K. Drugs, and the skin. In Clinical Pharmacology, 11th ed.; Bennett, P.N., Brown, M.J., Sharma, P., Eds.; Churchill Livingstone: London, UK, 2012; pp. 260–275. [Google Scholar]
- Li, X.; Yu, C.; Hu, Y.; Xia, X.; Liao, Y.; Zhang, J.; Chen, H.; Lu, W.; Zhou, W.; Song, Z. New Application of Psoralen and Angelicin on Periodontitis with Anti-bacterial, Anti-inflammatory, and Osteogenesis Effects. Front. Cell. Infect. Microbiol. 2018, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Fadus, M.C.; Lau, C.; Bikhchandani, J.; Lynch, H.T. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. J. Tradit. Complement. Med. 2016, 7, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Curcumin. 2019. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/curcumin (accessed on 13 April 2020).
- Das, S.; Das, D.K. Anti-inflammatory responses of resveratrol. Inflamm. Allergy Drug Targets 2007, 6, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Švajger, U.; Jeras, M. Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int. Rev. Immunol. 2012, 31, 202–222. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.M.; Zong, Y.; Sun, L.; Guo, J.Z.; Zhang, W.; He, Y.; Song, R.; Wang, W.M.; Xiao, C.J.; Lu, D. Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. PLoS ONE. 2012, 7, e32195. [Google Scholar] [CrossRef] [Green Version]
- Poulsen, M.M.; Fjeldborg, K.; Ornstrup, M.J.; Kjær, T.N.; Nøhr, M.K.; Pedersen, S.B. Resveratrol and inflammation: Challenges in translating pre-clinical findings to improved patient outcomes. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1124–1136. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, D.S.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef] [Green Version]
- BaGen, H.; Liu, X.; Han, J. The anti-inflammation effects of resveratrol for patients after oral implantology. Biomed. Res. 2018, 29, 1841–1844. [Google Scholar] [CrossRef] [Green Version]
- Chu, P.Y.; Hsu, D.Z.; Hsu, P.Y.; Liu, M.Y. Sesamol down-regulates the lipopolysaccharide-induced inflammatory response by inhibiting nuclear factor-kappa B activation. Innate Immun. 2010, 16, 333–339. [Google Scholar] [CrossRef]
- Yashaswini, P.S.; Rao, A.G.; Singh, S.A. Inhibition of lipoxygenase by sesamol corroborates its potential anti-inflammatory activity. Int. J. Biol. Macromol. 2017, 94, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Sá, R.C.S.; Andrade, L.N.; de Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar]
- Garcia, S.C.; Lopes, L.S.; Schott, K.L.; Beck, S.T.; Pomblum, V.J. Ciclosporina A and tacrolimus: Uma revisão. J. Bras. Patol. Med. Lab. 2004, 40, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Lemster, B.H.; Carroll, P.B.; Rilo, H.R.; Johnson, N.; Nikaein, A.; Thomson, A.W. IL-8/IL-8 receptor expression in psoriasis and the response to systemic tacrolimus (FK506) therapy. Clin. Exp. Immunol. 1995, 99, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Emal, D.; Rampanelli, E.; Claessen, N.; Bemelman, F.J.; Leemans, J.C.; Florquin, S.; Dessing, M.C. Calcineurin inhibitor Tacrolimus impairs host immune response against urinary tract infection. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Mu, H.; Holm, R. Solid lipid nanocarriers in drug delivery: Characterization and design. Expert Opin. Drug Deliv. 2018, 15, 771–785. [Google Scholar] [CrossRef]
- Pivetta, T.P.; Simões, S.; Araújo, M.M.; Carvalho, T.; Arruda, C.; Marcato, P.D. Development of nanoparticles from natural lipids for topical delivery of thymol: Investigation of its anti-inflammatory properties. Colloids Surf. B Biointerfaces 2018, 164, 281–290. [Google Scholar] [CrossRef]
- Puglia, C.; Bonina, F. Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals. Expert Opin. Drug Deliv. 2012, 9, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Wissing, S.A.; Barbosa, C.M.; Müller, R.H. Evaluation of the physical stability of SLN and NLC before and after incorporation into hydrogel formulations. Eur. J. Pharm. Biopharm. 2004, 58, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanap, G.S.; Mohanta, G.P. Investigation of the factors influencing the incorporation of miconazole in SNL and NLC dispersion. IAJPS 2014, 4, 1378–1390. [Google Scholar]
- Müller, R.H.; Lucks, J.S. Inventors. Arzneistoffträger aus Festen Lipidteilchen, Feste Lipidnanosphären (SLN). European Patent 0605497, 25 April 1996. [Google Scholar]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Kammari, R.; Das, N.G.; Das, S.K. Nanoparticulate Systems for Therapeutic and Diagnostic Applications. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Mitra, A., Cholkar, K., Mandal, A., Eds.; Elsevier: Kansas City, MO, USA, 2017; pp. 105–144. [Google Scholar]
- Zhai, Y.; Zhai, G. Advances in lipid-based colloid systems as drug carrier for topic delivery. J. Control. Release 2014, 193, 90–99. [Google Scholar] [CrossRef]
- Müller, R.H.; Petersen, R.D.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Ram, D.T.; Debnath, S.; Babu, M.N.; Nath, T.C.; Thejeswi, B. A review on solid lipid nanoparticles. RJPT 2012, 5, 1359–1368. [Google Scholar]
- Souto, E.B.; Müller, R.H. Cosmetic features, and applications of lipid nanoparticles (SLN, NLC). Int. J. Cosmet. Sci. 2008, 30, 157–165. [Google Scholar] [CrossRef]
- Jensen, L.B.; Petersson, K.; Nielsen, H.M. In vitro penetration properties of solid lipid nanoparticles in intact and barrier-impaired skin. Eur. J Pharm. Biopharm. 2011, 79, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Nirbhavane, P.; Sharma, G.; Singh, B.; Khuller, G.K.; Goni, V.G.; Patil, A.B.; Katare, O.P. Preclinical Explorative Assessment of Celecoxib-Based Biocompatible Lipidic Nanocarriers for the Management of CFA-Induced Rheumatoid Arthritis in Wistar Rats. AAPS PharmSciTech 2018, 19, 3187–3198. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.; Marques, C.; Figueiredo, J.L.; Gaio, A.R.; Costa, P.C.; Sousa Lobo, J.M.; Almeida, I.F. In vitro cytotoxicity evaluation of resveratrol-loaded nanoparticles: Focus on the challenges of in vitro methodologies. Food Chem. Toxicol. 2017, 103, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.; Lippacher, A.; Müller, R. Investigations on the occlusive properties of solid lipid nanoparticles (SLN). J. Cosmet. Sci. 2001, 52, 313–324. [Google Scholar]
- Charcosset, C.; El-Harati, A.; Fessi, H. Preparation of solid lipid nanoparticles using a membrane contactor. J. Control. Release 2005, 108, 112–120. [Google Scholar] [CrossRef]
- Fang, J.Y.; Fang, C.L.; Liu, C.H.; Su, Y.H. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2008, 70, 633–640. [Google Scholar] [CrossRef]
- Montenegro, L.; Panico, A.M.; Santagati, L.M.; Siciliano, E.A.; Intagliata, S.; Modica, M.N. Solid Lipid Nanoparticles Loading Idebenone Ester with Pyroglutamic Acid: In Vitro Antioxidant Activity and In Vivo Topical Efficacy. Nanomaterials 2018, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Pallerla, S.M.; Prabhakar, B.R. A Review on Solid Lipid Nanoparticles. Int. J. Pharm. Sci. Rev. Res. 2013, 20, 196–206. [Google Scholar]
- Rostamkalaei, S.S.; Akbari, J.; Saeedi, M.; Morteza-Semnani, K.; Nokhodchi, A. Topical gel of Metformin solid lipid nanoparticles: A hopeful promise as a dermal delivery system. Colloids Surf. B Biointerfaces 2019, 175, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Ruktanonchai, U.; Bejrapha, P.; Sakulkhu, U.; Opanasopit, P.; Bunyapraphatsara, N.; Junyaprasert, V.; Puttipipatkhachorn, S. Physicochemical characteristics, cytotoxicity, and antioxidant activity of three lipid nanoparticulate formulations of alpha-lipoic acid. AAPS PharmSciTech 2009, 10, 227–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.J.; Liu, K.S.; Sung, K.C.; Tsai, C.Y.; Fang, J.Y. Lipid nanoparticles with different oil/fatty ester ratios as carriers of buprenorphine and its prodrugs for injection. Eur. J. Pharm. Sci. 2009, 38, 138–146. [Google Scholar] [CrossRef]
- Soldati, P.P.; Polonini, H.C.; Paes, C.Q.; Restrepob, J.A.S.; Creczynksi-Pasa, T.B.; Chaves, M.G.A.M.; Brandão, M.A.F.; Pittella, F.; Raposo, N.R.B. Controlled release of resveratrol from lipid nanoparticles improves antioxidant effect. IFAC-PapersOnLine 2018, 51, 16–21. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization, and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Jenning, V.; Lippacher, A.; Gohla, S.H. Medium scale production of solid lipid nanoparticles (SLN) by high pressure homogenization. J. Microencapsul. 2002, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Martins-Gomes, C.; Ferreira, N.R.; Silva, A.M.; Nowak, I.; Souto, E.B. Anti-inflammatory and anti-cancer activity of citral: Optimization of citral-loaded solid lipid nanoparticles (SLN) using experimental factorial design and LUMiSizer®. Int. J. Pharm. 2018, 533, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Pizzol, C.D.; Filippin-Monteiro, F.B.; Restrepo, J.A.; Pittella, F.; Silva, A.H.; Alves de Souza, P.; Machado de Campos, A.; Creczynski-Pasa, T.B. Influence of surfactant and lipid type on the physicochemical properties and biocompatibility of solid lipid nanoparticles. Int. J. Environ. Res. Public Health 2014, 11, 8581–8596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigon, R.B.; Gonçalez, M.L.; Severino, P.; Alves, D.A.; Santana, M.H.A.; Souto, E.B.; Chorilli, M. Solid lipid nanoparticles optimized by 22 factorial design for skin administration: Cytotoxicity in NIH3T3 fibroblasts. Colloids Surf. B Biointerfaces 2018, 171, 501–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altube, M.J.; Cutro, A.; Bakas, L.; Morilla, M.J.; Disalvo, E.A.; Romero, E.L. Nebulizing novel multifunctional nanovesicles: The impact of macrophage-targeted-pH-sensitive archaeosomes on a pulmonary surfactant. J. Mater. Chem. B 2017, 5, 8083–8095. [Google Scholar] [CrossRef] [PubMed]
- Higa, L.H.; Jerez, H.E.; de Farias, M.A.; Portugal, R.V.; Romero, E.L.; Morilla, M.J. Ultra-small solid archaeolipid nanoparticles for active targeting to macrophages of the inflamed mucosa. Nanomedicine 2017, 12, 1165–1175. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Li, Y.; Wu, X.Y. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 491–504. [Google Scholar] [CrossRef]
- Zamarioli, C.M.; Martins, R.M.; Carvalho, E.C.; Freitas, L.A.P. Nanoparticles containing curcuminoids (Curcuma longa): Development of topical delivery formulation. Rev. Bras. Farmacogn. 2015, 25, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid lipid nanoparticles: Emerging colloidal nano drug delivery system. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Garud, A.; Singh, D.; Garud, N. Solid Lipid Nanoparticles (SLN): Method, Characterization and Applications. Int. Curr. Pharm. J. 2012, 1, 384–393. [Google Scholar] [CrossRef] [Green Version]
- Maa, Y.F.; Hsu, C.C. Performance of sonication and microfluidization for liquid-liquid emulsification. Pharm. Dev. Technol. 1999, 4, 233–240. [Google Scholar] [CrossRef]
- Hielscher, T. Ultrasonic Production of Nano-Size Dispersions and Emulsions. In Proceedings of the 5th ENS@T Scientific Meeting, Paris, France, 9–10 December 2005; EDA Publishing Association: Paris, France, 2005; pp. 138–143. [Google Scholar]
- Cavalli, R.; Caputo, O.; Carlotti, M.E.; Trotta, M.; Scarnecchia, C.; Gasco, M.R. Sterilization and freeze-drying of drug-free and drug-loaded solid lipid nanoparticles. Int. J. Pharm. 1997, 148, 47–54. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; A Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leskošek-Čukalović, I.J.; Despotović, S.M.; Nedović, V.A.; Nikšić, M.P. Medicinal mushroom Ganoderma lucidum in the production of special beer types. Zb. Matice Srp. Prir. Nauk. 2009, 117, 111–117. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; D’Ambrosio, M.; Luceri, C.; Cirri, M. Evaluation and comparison of Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) as vectors to develop hydrochlorothiazide effective and safe pediatric oral liquid formulations. Pharmaceutics 2021, 13, 437. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Goulart, G.C.A.; Ferreira, L.A.M.; Carneiro, G. Hydrophobic ion pairing as a strategy to improve drug encapsulation into lipid nanocarriers for the cancer treatment. Expert Opin. Drug Deliv. 2017, 14, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, G.K.; Carvalho, E.L.S.; Poser, G.L.; Teixeira, H.F. On the use of nanotechnology-based strategies for association of complex matrices from plants extracts. Rev. Bras. Farmacogn. 2015, 25, 426–436. [Google Scholar] [CrossRef]
- Subramaniam, B.; Siddik, Z.H.; Nagoor, N.H. Optimization of nanostructured lipid carriers: Understanding the types, designs, and parameters in the process of formulations. J. Nanopart. Res. 2020, 22, 141. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; Pino, V. Extraction with ionic liquids-organic compounds. In Liquid-Phase Extraction; Poole, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 499–537. [Google Scholar]
- Radünz, M.; Hackbart, H.C.S.; Camargo, T.M.; Nunes, C.F.P.; de Barros, F.A.P.; Dal Magro, J.D.; Sanchez Filho, P.J.; Gandra, E.A.; Radünz, A.L.; Zavareze, E.R. Antimicrobial potential of spray drying encapsulated thyme (Thymus vulgaris) essential oil on the conservation of hamburger-like meat products. Intern. J. Food Microbiol. 2020, 330, 108696. [Google Scholar] [CrossRef]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055–6074. [Google Scholar]
- Kim, S.T.; Jang, D.J.; Kim, J.H.; Park, J.Y.; Lim, J.S.; Lee, S.Y.; Lee, K.M.; Lim, S.J.; Kim, C.K. Topical administration of cyclosporin A in a solid lipid nanoparticle formulation. Pharmazie 2009, 64, 510–514. [Google Scholar] [PubMed]
- Essaghraoui, A.; Belfkira, A.; Hamdaoui, B.; Nunes, C.; Lima, S.A.C.; Reis, S. Improved Dermal Delivery of Cyclosporine A Loaded in Solid Lipid Nanoparticles. Nanomaterials 2019, 9, 1204. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.I.; Barbosa, A.I.; Costa Lima, S.A.; Costa, P.; Torres, T.; Reis, S. Freeze-Dried Softisan® 649-Based Lipid Nanoparticles for Enhanced Skin Delivery of Cyclosporine, A. Nanomaterials 2020, 10, 986. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Servidio, C.; Laganà, A.S.; Conforti, F.; Marrelli, M.; Cassano, R. Viscosified Solid Lipidic Nanoparticles Based on Naringenin and Linolenic Acid for the Release of Cyclosporine A on the Skin. Molecules 2020, 25, 3535. [Google Scholar] [CrossRef]
- Arora, R.; Katiyar, S.S.; Kushwah, V.; Jain, S. Solid lipid nanoparticles and nanostructured lipid carrier-based nanotherapeutics in treatment of psoriasis: A comparative study. Expert Opin. Drug Deliv. 2017, 14, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Caon, T.; Mazzarino, L.; Simões, C.M.; Senna, E.L.; Silva, M.A. Lipid- and Polymer-Based Nanostructures for Cutaneous Delivery of Curcumin. AAPS PharmSciTech 2017, 18, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Iriventi, P.; Gupta, N.V. Topical delivery of curcumin and caffeine mixture-loaded nanostructured lipid carriers for effective treatment of psoriasis. Pharmacogn. Mag. 2020, 16, 206–217. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lipid nanocarriers for dermal delivery of lutein: Preparation, characterization, stability, and performance. Int. J. Pharm. 2011, 414, 267–275. [Google Scholar] [CrossRef]
- Okonogi, S.; Riangjanapatee, P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int. J. Pharm. 2015, 478, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Michniak-Kohn, B. Preparation and characterization of lipid based nanosystems for topical delivery of quercetin. Eur. J. Pharm. Sci. 2013, 48, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhao, G.; Ni, S.; Xia, Q. Lipid based nanocarriers with different lipid compositions for topical delivery of resveratrol: Comparative analysis of characteristics and performance. J. Drug Deliv. Sci. Technol. 2014, 24, 591–600. [Google Scholar] [CrossRef]
- Viegas, J.S.R.; Praca, F.G.; Caron, A.L.; Suzuki, I.; Silvestrini, A.V.P.; Medina, W.S.G.; Ciampo, J.O.D.; Kravicz, M.; Bentley, M.V.L.B. Nanostructured lipid carrier co-delivering tacrolimus and TNF-α siRNA as an innovate approach to psoriasis. Drug Deliv. Transl. Res. 2020, 10, 646–660. [Google Scholar] [CrossRef]
- Gárcia-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [Green Version]
- Thurnham, D.I.; Northrop-Clewes, C.A. Inflammation and biomarkers of micronutrient status. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 458–463. [Google Scholar] [CrossRef]
- Ford, E.S.; Liu, S.; Mannino, D.M.; Giles, W.H.; Smith, S.J. C-reactive protein concentration and concentrations of blood vitamins, carotenoids, and selenium among United States adults. Eur. J. Clin. Nutr. 2003, 57, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Lacatusu, I.; Badea, G.; Popescu, M.; Bordei, N.; Istrati, D.; Moldovan, L.; Seciu, A.M.; Panteli, M.I.; Rasit, I.; Badea, N. Marigold extract, azelaic acid and black caraway oil into lipid nanocarriers provides a strong anti-inflammatory effect in vivo. Ind. Crop. Prod. 2017, 109, 141–150. [Google Scholar] [CrossRef]
- Faiyazuddin, M.; Akhtar, N.; Akhter, J.; Suri, S.; Shakeel, F.; Shafiq, S.; Mustafa, G. Production, characterization, in vitro and ex vivo studies of babchi oil-encapsulated nanostructured solid lipid carriers produced by a hot aqueous titration method. Pharmazie 2010, 65, 348–355. [Google Scholar]
- Varman, R.M.; Singh, S. Investigation of effects of terpene skin penetration enhancers on stability and biological activity of lysozyme. AAPS PharmSciTech 2012, 13, 1084–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. The Anti-Inflammatory Properties of Terpenoids from Cannabis. Cannabis Cannabinoid Res. 2018, 3, 282–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afra, B.; Mohammadi, M.; Soleimani, M.; Mahjub, R. Preparation, Statistical Optimization, In Vitro Characterization, and In Vivo Pharmacological Evaluation of Solid Lipid Nanoparticles Encapsulating Propolis Flavonoids: A Novel Treatment for Skin Edema. Drug Dev. Ind. Pharm. 2020, 46, 1–51. [Google Scholar] [CrossRef]







| Chemical Group | Examples of Substances | Mechanism of Action as an Anti-Inflammatory |
|---|---|---|
| Carotenoids | Fucoxanthin | Acts by restraining tyrosinase activity [61] and nitric oxide production. It also inhibits nitric oxide synthase, cyclooxygenase 2 (COX-2), and prostaglandin protein expressions. In the same way, TNF-α, IL-1β, and IL-6 are reduced after fucoxanthin treatment [62]. |
| Lutein | Decreases pro-inflammatory cytokines such as IL-6, IL-1β, and TNF [63,64]. Inhibits cyclooxygenase expression [63], which downregulates the activation of prostaglandin [65]. Acts through radical scavenging activity by AP-1 pathway [63]. | |
| Lycopene | Inhibits pro-inflammatory proteins, such as TNFα, IL-1β, IL-6, and IL-8, by the NF-kβ pathway and induces the expression of anti-inflammatory cytokines, such as IL-10 [66]. Presents antioxidant activity due to its eleven conjugated double bonds [67]. Inhibits redox by suppressing ROS-producing enzymes like cyclooxygenase, lipoxygenase, nitric oxide synthase, and NADPH oxidase [68]. | |
| Flavonoids | Quercetin | Suppresses pro-inflammatory pathways, such as AP-1, cyclooxygenase, and NF-kβ, therefore, inhibiting several pro-inflammatory cytokines, as TNF-α and IL-1β [69] and iNOS [70]. It also inhibits xanthine oxidase and lipoxygenase, decreasing oxidative injury [71], and quinone reductase 2 that catalyzes toxic compounds, forming ROS [72]. |
| Furocoumarin | Psoralen | Inhibits cell division and proliferation through DNA interaction [73]. Decreases the levels of pro-inflammatory cytokines, such as IL-1β [74]. |
| Phenolics | Curcumin | Produces anti-inflammatory effect through the peroxisome proliferator-activated receptor gamma (PPAR-y) pathway [75]. Causes the reduction of NF-kβ and AP-1 pathways, which inhibits pro-inflammatory mediators, such as TNF-α and other cytokines [75,76]. Blocks the formation of ROS and the production of pro-inflammatory cytokines by inhibiting cyclooxygenase [77]. Scavenges reactive species, modulates the activity of glutathione peroxidase, catalase, and superoxide dismutase, besides inhibiting ROS-generating enzymes such as cyclooxygenase, and also lipoxygenase, and xanthine hydrogenase [76]. |
| Resveratrol | Inhibits the AP-1 and NF-kβ pathways [78,79]. Blocks the expression of cyclooxygenase and cytokines, such as IL-1, IL-8, iNOS, and TNF-α [78,80,81,82]. Upregulates anti-inflammatory cytokines, such as IL-2 and IL-10 [83]. Scavenges the reactive oxygen species [78]. | |
| Sesamol | Inhibits cytokine production of TNF-α and IL-1β by suppressing the NF-kB pathway [84]. Inhibits lipoxygenase through its radical scavenging activity, due to the presence of a benzodiol group [85]. | |
| Terpenoids | Thymol | Inhibits cyclooxygenase. Inactivates calcium channels by triggering the reduction of elastase [86]. |
| Natural Compound. | Biological Activity | Limitations | Results |
|---|---|---|---|
| Cyclosporin A [138,139,140,141,142] | Immunosuppressant |
|
|
| Curcumin [26,123,143,144] and curcuminoids [21,123] | Anti-inflammatory and antioxidant |
|
|
| Fucoxanthin [61] | Anti-inflammatory and antioxidant |
|
|
| Lutein [145] | Anti-inflammatory and antioxidant |
|
|
| Lycopene [146] | Antioxidant and anti-inflammatory |
|
|
| Psoralen [107] | Anti-inflammatory and anti-proliferative |
|
|
| |||
| Quercetin [20,147] | Anti-inflammatory and antioxidant |
|
|
| Resveratrol [113,148] | Antioxidant and anti-inflammatory |
|
|
| Sesamol [17] | Anti-inflammatory and antioxidant |
|
|
| Tacrolimus [149] | Immunosuppressant |
|
|
| Thymol [91] | Anti-inflammatory and antioxidant |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, K.C.B.; Valle, A.B.C.d.S.; Paes, C.Q.; Tavares, G.D.; Pittella, F. Nanostructured Lipid Carriers for the Formulation of Topical Anti-Inflammatory Nanomedicines Based on Natural Substances. Pharmaceutics 2021, 13, 1454. https://doi.org/10.3390/pharmaceutics13091454
Ferreira KCB, Valle ABCdS, Paes CQ, Tavares GD, Pittella F. Nanostructured Lipid Carriers for the Formulation of Topical Anti-Inflammatory Nanomedicines Based on Natural Substances. Pharmaceutics. 2021; 13(9):1454. https://doi.org/10.3390/pharmaceutics13091454
Chicago/Turabian StyleFerreira, Kézia Cristine Barbosa, Ana Beatriz Caribé dos Santos Valle, Camila Quinetti Paes, Guilherme Diniz Tavares, and Frederico Pittella. 2021. "Nanostructured Lipid Carriers for the Formulation of Topical Anti-Inflammatory Nanomedicines Based on Natural Substances" Pharmaceutics 13, no. 9: 1454. https://doi.org/10.3390/pharmaceutics13091454