Cryogenic Electron Microscopy Methodologies as Analytical Tools for the Study of Self-Assembled Pharmaceutics
Abstract
:1. Introduction
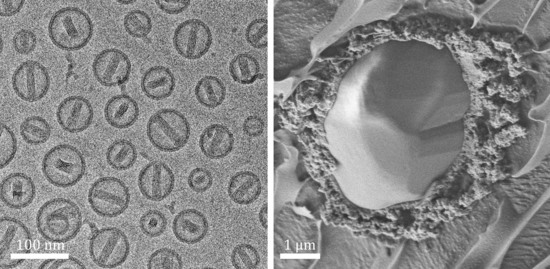
2. Cryogenic-Temperature Electron Microscopy
2.1. Cryogenic-Temperature Transmission Electron Microscopy (Cryo-TEM)
2.2. Cryogenic-Temperature Scanning Electron Microcopy (Cryo-SEM)
3. Examples of Cryo-EM Applications
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hernandez, C.; Gulati, S.; Fioravanti, G.; Stewart, P.L.; Exner, A.A. Cryo-EM Visualization of Lipid and Polymer-Stabilized Perfluorocarbon Gas Nanobubbles–A Step Towards Nanobubble Mediated Drug Delivery. Sci. Rep. 2017, 7, 13157. [Google Scholar] [CrossRef] [Green Version]
- Erlich, M.; Arie, T.; Koifman, N.; Talmon, Y. Structure elucidation of silica-based core–shell microencapsulated drugs for topical applications by cryogenic scanning electron microscopy. J. Colloid Interface Sci. 2020, 579, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Villa, F.; Quarto, R.; Tasso, R. Extracellular vesicles as natural, safe and efficient drug delivery systems. Pharmaceutics 2019, 11, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, O.G.; Kooijmans, S.A.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.W.; Sluijter, J.P.G.; Vader, P.; Schiffelers, R.M. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.L.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Stevens, M.M. Strategic design of extracellular vesicle drug delivery systems. Adv. Drug Deliv. Rev. 2018, 130, 12–16. [Google Scholar] [CrossRef]
- Issman, L.; Brenner, B.; Talmon, Y.; Aharon, A. Cryogenic Transmission Electron Microscopy Nanostructural Study of Shed Microparticles. PLoS ONE 2013, 8, e83680. [Google Scholar] [CrossRef] [Green Version]
- Koifman, N.; Biran, I.; Aharon, A.; Brenner, B.; Talmon, Y. A direct-imaging cryo-EM study of shedding extracellular vesicles from leukemic monocytes. J. Struct. Biol. 2017, 198, 177–185. [Google Scholar] [CrossRef]
- Matthies, D.; Lee, N.Y.J.; Gatera, I.; Pasolli, H.A.; Zhao, X.; Liu, H.; Walpita, D.; Liu, Z.; Yu, Z.; Ioannou, M.S. Microdomains form on the luminal face of neuronal extracellular vesicle membranes. Sci. Rep. 2020, 10, 11953. [Google Scholar] [CrossRef]
- Busatto, S.; Yang, Y.; Walker, S.A.; Davidovich, I.; Lin, W.H.; Lewis-Tuffin, L.; Anastasiadis, P.Z.; Sarkaria, J.; Talmon, Y.; Wurtz, G.; et al. Brain metastases-derived extracellular vesicles induce binding and aggregation of low-density lipoprotein. J. Nanobiotechnol. 2020, 18. [Google Scholar] [CrossRef]
- Tian, M.; Ticer, T.; Wang, Q.; Walker, S.; Pham, A.; Suh, A.; Busatto, S.; Davidovich, I.; Al-Kharboosh, R.; Lewis-Tuffin, L.; et al. Adipose-Derived Biogenic Nanoparticles for Suppression of Inflammation. Small 2020, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oieni, J.; Levy, L.; Letko Khait, N.; Yosef, L.; Schoen, B.; Fliman, M.; Shalom-Luxenburg, H.; Malkah Dayan, N.; D’Atri, D.; Cohen Anavy, N.; et al. Nano-Ghosts: Biomimetic membranal vesicles, technology and characterization. Methods 2020, 177, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Van Drie, J.H.; Tong, L. Cryo-EM as a powerful tool for drug discovery. Bioorg. Med. Chem. Lett. 2020, 30, 127524. [Google Scholar] [CrossRef]
- Wyllie, S.; Brand, S.; Thomas, M.; De Rycker, M.; Chung, C.; Pena, I.; Bingham, R.P.; Bueren-Calabuig, J.A.; Cantizani, J.; Cebrian, D.; et al. Preclinical candidate for the treatment of visceral leishmaniasis that acts through proteasome inhibition. Proc. Natl. Acad. Sci. USA 2019, 116, 9318–9323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceska, T.; Chung, C.W.; Cooke, R.; Phillips, C.; Williams, P.A. Cryo-EM in drug discovery. Biochem. Soc. Trans. 2019, 47, 281–293. [Google Scholar] [CrossRef] [Green Version]
- Renaud, J.P.; Chari, A.; Ciferri, C.; Liu, W.T.; Rémigy, H.W.; Stark, H.; Wiesmann, C. Cryo-EM in drug discovery: Achievements, limitations and prospects. Nat. Rev. Drug Discov. 2018, 17, 471–492. [Google Scholar] [CrossRef]
- Ke, Z.; Oton, J.; Qu, K.; Cortese, M.; Zila, V.; Mckeane, L.; Nakane, T.; Zivanov, J.; Neufeldt, C.J.; Cerikan, B.; et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature 2020, 588. [Google Scholar] [CrossRef]
- Ismail, A.M.; Elfiky, A.A. SARS-CoV-2 spike behavior in situ: A Cryo-EM images for a better understanding of the COVID-19 pandemic. Signal Transduct. Target. Ther. 2020, 5, 252. [Google Scholar] [CrossRef]
- Liang, Y.L.; Khoshouei, M.; Radjainia, M.; Zhang, Y.; Glukhova, A.; Tarrasch, J.; Thal, D.M.; Furness, S.G.B.; Christopoulos, G.; Coudrat, T.; et al. Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature 2017, 546, 118–123. [Google Scholar] [CrossRef]
- Jejurikar, A.; Seow, X.T.; Lawrie, G.; Martin, D.; Jayakrishnan, A.; Grøndahl, L. Degradable alginate hydrogels crosslinked by the macromolecular crosslinker alginate dialdehyde. J. Mater. Chem. 2012, 22, 9751–9758. [Google Scholar] [CrossRef]
- Straccia, M.; d’Ayala, G.; Romano, I.; Oliva, A.; Laurienzo, P. Alginate Hydrogels Coated with Chitosan for Wound Dressing. Mar. Drugs 2015, 13, 2890–2908. [Google Scholar] [CrossRef] [Green Version]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Fabrication and Characterization of Hydrogels Based on Gelatinised Collagen with Potential Application in Tissue Engineering. Polymers 2020, 12, 1146. [Google Scholar] [CrossRef]
- Aston, R.; Sewell, K.; Klein, T.; Lawrie, G.; Grøndahl, L. Evaluation of the impact of freezing preparation techniques on the characterisation of alginate hydrogels by cryo-SEM. Eur. Polym. J. 2016, 82, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Marmorat, C.; Arinstein, A.; Koifman, N.; Talmon, Y.; Zussman, E.; Rafailovich, M. Cryo-Imaging of Hydrogels Supermolecular Structure. Sci. Rep. 2016, 6, 25495. [Google Scholar] [CrossRef] [Green Version]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Sharma, U.S. Liposomes in drug delivery: Progress and limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar] [CrossRef]
- Baxa, U. Imaging of liposomes by transmission electron microscopy. In Methods in Molecular Biology; Humana Press Inc.: New York, NY, USA, 2018; Volume 1682, pp. 73–88. [Google Scholar]
- Almgren, M.; Edwards, K.; Karlsson, G. Cryo transmission electron microscopy of liposomes and related structures. Colloids Surf. A Physicochem. Eng. Asp. 2000, 174, 3–21. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®–The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Yaari, Z.; Da Silva, D.; Zinger, A.; Goldman, E.; Kajal, A.; Tshuva, R.; Barak, E.; Dahan, N.; Hershkovitz, D.; Goldfeder, M.; et al. Theranostic barcoded nanoparticles for personalized cancer medicine. Nat. Commun. 2016, 7, 13325. [Google Scholar] [CrossRef] [Green Version]
- Spernath, A.; Aserin, A. Microemulsions as carriers for drugs and nutraceuticals. Adv. Colloid Interface Sci. 2006, 128–130, 47–64. [Google Scholar] [CrossRef]
- Jha, S.K.; Karki, R. Microemulsions-Potential Carrier for Improved Drug Delivery. Asian J. Biomed. Pharm. Sci. 2011, 1, 5–9. [Google Scholar]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Wolf, L.; Hoffmann, H.; Talmon, Y.; Teshigawara, T.; Watanabe, K. Cryo-TEM imaging of a novel microemulsion system of silicone oil with an anionic/nonionic surfactant mixture. Soft Matter 2010, 6, 5367–5374. [Google Scholar] [CrossRef]
- Wolf, L.; Hoffmann, H.; Teshigawara, T.; Okamoto, T.; Talmon, Y. Microemulsions with a HIPME (high internal phase microemulsion) structure. J. Phys. Chem. B 2012, 116, 2131–2137. [Google Scholar] [CrossRef]
- Ben-Barak, I.; Talmon, Y. Direct-Imaging Cryo-SEM of Nanostructure Evolution in Didodecyldimethylammonium Bromide-Based Microemulsions. Z. Phys. Chem. 2012, 226, 665–674. [Google Scholar] [CrossRef]
- Davidovich, I.; Issman, L.; de Paula, C.; Ben-Barak, I.; Talmon, Y. A cryogenic-electron microscopy study of the one-phase corridor in the phase diagram of a nonionic surfactant-based microemulsion system. Colloid Polym. Sci. 2015, 293, 3189–3197. [Google Scholar] [CrossRef]
- Gradzielski, M.; Duvail, M.; de Molina, P.M.; Simon, M.; Talmon, Y.; Zemb, T. Using Microemulsions: Formulation Based on Knowledge of Their Mesostructure. Chem. Rev. 2021, 121, 5671–5740. [Google Scholar] [CrossRef]
- Murgia, S.; Falchi, A.M.; Mano, M.; Lampis, S.; Angius, R.; Carnerup, A.M.; Schmidt, J.; Diaz, G.; Giacca, M.; Talmon, Y.; et al. Nanoparticles from lipid-based liquid crystals: Emulsifier influence on morphology and cytotoxicity. J. Phys. Chem. B 2010, 114, 3518–3525. [Google Scholar] [CrossRef] [PubMed]
- Murgia, S.; Bonacchi, S.; Falchi, A.M.; Lampis, S.; Lippolis, V.; Meli, V.; Monduzzi, M.; Prodi, L.; Schmidt, J.; Talmon, Y.; et al. Drug-loaded fluorescent cubosomes: Versatile nanoparticles for potential theranostic applications. Langmuir 2013, 29, 6673–6679. [Google Scholar] [CrossRef]
- Siegel, D.P.; Burns, J.L.; Chestnut, M.H.; Talmon, Y. Intermediates in membrane fusion and bilayer/nonbilayer phase transitions imaged by time-resolved cryo-transmission electron microscopy. Biophys. J. 1989, 56, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Szebeni, J.; Alving, C.R.; Savay, S.; Barenholz, Y.; Priev, A.; Danino, D.; Talmon, Y. Formation of complement-activating particles in aqueous solutions of Taxol: Possible role in hypersensitivity reactions. Int. Immunopharmacol. 2001, 1, 721–735. [Google Scholar] [CrossRef]
- Attili-Qadri, S.; Karra, N.; Nemirovski, A.; Schwob, O.; Talmon, Y.; Nassar, T.; Benita, S. Oral delivery system prolongs blood circulation of docetaxel nanocapsules via lymphatic absorption. Proc. Natl. Acad. Sci. USA 2013, 110, 17498–17503. [Google Scholar] [CrossRef] [Green Version]
- Kuntsche, J.; Horst, J.C.; Bunjes, H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int. J. Pharm. 2011, 417, 120–137. [Google Scholar] [CrossRef]
- Talmon, Y. Staining and drying-induced artifacts in electron microscopy of surfactant dispersions. J. Colloid Interface Sci. 1983, 93, 366–382. [Google Scholar] [CrossRef]
- Waisman, D.; Danino, D.; Weintraub, Z.; Schmidt, J.; Talmon, Y. Nanostructure of the aqueous form of lung surfactant of different species visualized by cryo-transmission electron microscopy. Clin. Physiol. Funct. Imaging 2007, 27, 375–380. [Google Scholar] [CrossRef]
- Adrian, M.; Dubochet, J.; Lepault, J.; McDowall, A.W. Cryo-electron microscopy of viruses. Nature 1984, 308, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Hodgdon, T.K.; Kaler, E.W.; Abezgauz, L.; Danino, D.; Lubovsky, M.; Talmon, Y.; Pochan, D.J.; Bellare, J.R.; Davis, H.T.; et al. Elucidating the assembled structure of amphiphiles in solution via cryogenic transmission electron microscopy. Soft Matter 2007, 3, 945. [Google Scholar] [CrossRef] [PubMed]
- Talmon, Y. Transmission Electron Microscopy of Complex Fluids: The State of the Art. Ber. Bunsenges. Phys. Chem. 1996, 100, 364–372. [Google Scholar] [CrossRef]
- Talmon, Y. The study of nanostructured liquids by cryogenic-temperature electron microscopy–A status report. J. Mol. Liq. 2015, 210, 2–8. [Google Scholar] [CrossRef]
- Siegel, D.P.; Epand, R.M. The mechanism of lamellar-to-inverted hexagonal phase transitions in phosphatidylethanolamine: Implications for membrane fusion mechanisms. Biophys. J. 1997, 73, 3089–3111. [Google Scholar] [CrossRef] [Green Version]
- Oostergetel, G.T.; Esselink, F.J.; Hadziioannou, G. Cryo-Electron Microscopy of Block Copolymers in an Organic Solvent. Langmuir 1995, 11, 3721–3724. [Google Scholar] [CrossRef]
- Bellare, J.R.; Davis, H.T.; Scriven, L.E.; Talmon, Y. Controlled environment vitrification system: An improved sample preparation technique. J. Electron Microsc. Tech. 1988, 10, 87–111. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, Z.; Zakin, J.L.; Talmon, Y.; Davis, H.T.; Scriven, L.E. Cryo-TEM Imaging the Flow-Induced Transition from Vesicles to Threadlike Micelles. J. Phys. Chem. B 2000, 104, 5263–5271. [Google Scholar] [CrossRef]
- Talmon, Y.; Narkis, M.; Silverstein, M. Electron beam radiation damage to organic inclusions in ice as an analytical tool for polymer science. J. Electron Microsc. Tech. 1985, 2, 589–596. [Google Scholar] [CrossRef]
- Liberman, L.; Kleinerman, O.; Davidovich, I.; Talmon, Y. Micrograph contrast in low-voltage SEM and cryo-SEM. Ultramicroscopy 2020, 218, 113085. [Google Scholar] [CrossRef]
- Patterson, J.P.; Xu, Y.; Moradi, M.A.; Sommerdijk, N.A.J.M.; Friedrich, H. CryoTEM as an Advanced Analytical Tool for Materials Chemists. Acc. Chem. Res. 2017, 50, 1495–1501. [Google Scholar] [CrossRef]
- Oss-Ronen, L.; Schmidt, J.; Abetz, V.; Radulescu, A.; Cohen, Y.; Talmon, Y. Characterization of block copolymer self-assembly: From solution to nanoporous membranes. Macromolecules 2012, 45, 9631–9642. [Google Scholar] [CrossRef]
- Matatyaho Ya’akobi, A.; Talmon, Y. Extending Cryo-EM to Nonaqueous Liquid Systems. Acc. Chem. Res. 2021, 54, 2100–2109. [Google Scholar] [CrossRef]
- Kleinerman, O.; Parra-Vasquez, A.N.G.; Green, M.J.; Behabtu, N.; Schmidt, J.; Kesselman, E.; Young, C.C.; Cohen, Y.; Pasquali, M.; Talmon, Y. Cryogenic-temperature electron microscopy direct imaging of carbon nanotubes and graphene solutions in superacids. J. Microsc. 2015, 259, 16–25. [Google Scholar] [CrossRef] [PubMed]
- EM GP2 Automatic Plunge Freezing|Products|Leica Microsystems. Available online: https://www.leica-microsystems.com/products/sample-preparation-for-electron-microscopy/p/leica-em-gp2 (accessed on 21 May 2021).
- Vitrobot for Life Sciences | Thermo Fisher Scientific. Available online: https://www.fei.com/products/vitrobot/#gsc.tab=0 (accessed on 21 May 2021).
- Danev, R.; Nagayama, K. Single particle analysis based on Zernike phase contrast transmission electron microscopy. J. Struct. Biol. 2008, 161, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Issman, L.; Talmon, Y. Cryo-SEM Specimen Preparation under Controlled Temperature and Concentration Conditions. J. Microsc. 2012, 246, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Studer, D.; Humbel, B.M.; Chiquet, M. Electron microscopy of high pressure frozen samples: Bridging the gap between cellular ultrastructure and atomic resolution. Histochem. Cell Biol. 2008, 130, 877–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Studer, D.; Graber, W.; Al-Amoudi, A.; Eggli, P. A new approach for cryofixation by high-pressure freezing. J. Microsc. 2001, 203, 285–294. [Google Scholar] [CrossRef] [PubMed]
- LEICA EM ICE High Pressure Freezer. Available online: https://www.leica-microsystems.com/products/sample-preparation-for-electron-microscopy/p/leica-em-ice/ (accessed on 21 May 2021).
- A breakthrough in sample preparation yield, simplicity and speed in high end cryo coating Freeze Fracture System LEICA EM ACE900. Available online: https://www.leica-microsystems.com/products/sample-preparation-for-electron-microscopy/p/leica-em-ace900/ (accessed on 21 May 2021).
- Bachmann, L.; Talmon, Y. Cryomicroscopy of liquid and semiliquid specimens: Direct imaging versus replication. Ultramicroscopy 1984, 14, 211–218. [Google Scholar] [CrossRef]
- Jacoby, G.; Cohen, K.; Barkan, K.; Talmon, Y.; Peer, D.; Beck, R. Metastability in lipid based particles exhibits temporally deterministic and controllable behavior. Sci. Rep. 2015, 5, 9481. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koifman, N.; Talmon, Y. Cryogenic Electron Microscopy Methodologies as Analytical Tools for the Study of Self-Assembled Pharmaceutics. Pharmaceutics 2021, 13, 1015. https://doi.org/10.3390/pharmaceutics13071015
Koifman N, Talmon Y. Cryogenic Electron Microscopy Methodologies as Analytical Tools for the Study of Self-Assembled Pharmaceutics. Pharmaceutics. 2021; 13(7):1015. https://doi.org/10.3390/pharmaceutics13071015
Chicago/Turabian StyleKoifman, Na’ama, and Yeshayahu Talmon. 2021. "Cryogenic Electron Microscopy Methodologies as Analytical Tools for the Study of Self-Assembled Pharmaceutics" Pharmaceutics 13, no. 7: 1015. https://doi.org/10.3390/pharmaceutics13071015