Synthesis and Evaluation of Novel Norfloxacin Isonitrile 99mTc Complexes as Potential Bacterial Infection Imaging Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Norfloxacin Isonitriles (CN4NF and CN5NF)
2.3. Radiolabeling
2.4. Stability Study
2.5. Partition Coefficient Measurement
2.6. In Vitro Bacterial Binding Study
2.7. Biodistribution Study in Bacteria-Infected Mice
2.8. Biodistribution Study in Turpentine-Induced Abscess Mice
2.9. SPECT Imaging
2.10. Statistical and Data Analysis
3. Results
3.1. Synthesis
3.2. Radiolabeling
3.3. Physicochemical Properties Evaluation
3.3.1. Stability Tests
3.3.2. Partition Coefficients
3.4. In Vitro Binding of [99mTc]Tc-CN4NF and [99mTc]Tc-CN5NF with Bacteria
3.5. Biodistribution Assessment
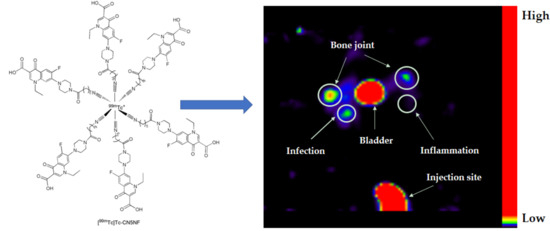
3.6. SPECT/CT Imaging Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirshojaei, S.F. Advances in infectious foci imaging using 99mTc radiolabelled antibiotics. J. Radioanal. Nucl. Chem. 2015, 304, 975–988. [Google Scholar] [CrossRef]
- Mota, F.; Ordonez, A.A.; Firth, G.; Ruiz-Bedoya, C.A.; Ma, M.T.; Jain, S.K. Radiotracer development for bacterial imaging. J. Med. Chem. 2020, 63, 1964–1977. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.; D’Alessandria, C.; Lazzeri, E.; Dierckx, R. Can we produce an image of bacteria with radiopharmaceuticals? Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1051–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, A.V.; Solanki, K.K.; Vinjamuri, S.; Britton, K.E.; Das, S.S. Evaluation of the efficacy of 99mTc-infection, a novel agent for detecting sites of infection. J. Clin. Pathol. 1998, 51, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.K. The promise of molecular imaging in the study and treatment of infectious diseases. Mol. Imaging Biol. 2017, 19, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Paez, D.; Orellana, P.; Gutierrez, C.; Ramirez, R.; Mut, F.; Torres, L. Current status of nuclear medicine practice in Latin America and the Caribbean. J. Nucl. Med. 2015, 56, 1629–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutton, B.F.; Erlandsson, K.; Thielemans, K. Advances in clinical molecular imaging instrumentation. Clin. Trans. Imaging 2018, 6, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Welling, M.M.; Hensbergen, A.W.; Bunschoten, A.; Velders, A.H.; Roestenberg, M.; van Leeuwen, F.W.B. An update on radiotracer development for molecular imaging of bacterial infections. Clin. Trans. Imaging 2019, 7, 105–124. [Google Scholar] [CrossRef] [Green Version]
- Streule, K.; Deschrijver, M.; Fridrich, R. 99mTc-labeled HSA-nanocolloid versus 111In-oxine-labeled granulocytes in detecting skeletal septic process. Nucl. Med. Commun. 1988, 9, 59–67. [Google Scholar] [CrossRef]
- Familiari, D.; Glaudemans, A.W.J.M.; Vitale, V.; Prosperi, D.; Bagni, O.; Lenza, A.; Cavallini, M.; Scopinaro, F.; Signore, A. Can sequential 18F-FDG PET/CT replace WBC imaging in the diabetic foot? J. Nucl. Med. 2011, 52, 1012–1019. [Google Scholar] [CrossRef] [Green Version]
- Ioppolo, J.A.; Caldwell, D.; Beiraghi, O.; Llano, L.; Blacker, M.; Valliant, J.F.; Berti, P.J. 67Ga-labeled deferoxamine derivatives for imaging bacterial infection: Preparation and screening of functionalized siderophore complexes. Nucl. Med. Biol. 2017, 52, 32–41. [Google Scholar] [CrossRef]
- Petrik, M.; Umlaufova, E.; Raclavsky, V.; Palyzova, A.; Havlicek, V.; Pfister, J.; Mair, C.; Novy, Z.; Popper, M.; Hajduch, M.; et al. 68Ga-labelled desferrioxamine-B for bacterial infection imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Pfister, J.; Summer, D.; Petrik, M.; Khoylou, M.; Lichius, A.; Kaeopookum, P.; Kochinke, L.; Orasch, T.; Haas, H.; Decristoforo, C. Hybrid imaging of Aspergillus fumigatus pulmonary infection with fluorescent, 68Ga-Labelled siderophores. Biomolecules 2020, 10, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renick, P.J.; Mulgaonkar, A.; Co, C.M.; Wu, C.-Y.; Zhou, N.; Velazquez, A.; Pennington, J.; Sherwood, A.; Dong, H.; Castellino, L.; et al. Imaging of actively proliferating bacterial infections by targeting the bacterial metabolic footprint with d-[5-11C]-glutamine. ACS Infect. Dis. 2021, 7, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Daryaee, F.; Yoon, G.E.; Noh, D.; Smith-Jones, P.M.; Si, Y.; Walker, S.G.; Turkman, N.; Meimetis, L.; Tonge, P.J. Positron emission tomography imaging of Staphylococcus aureus infection using a nitro-prodrug analogue of 2-[18F]F-p-aminobenzoic acid. ACS Infect. Dis. 2020, 6, 2249–2259. [Google Scholar] [CrossRef]
- Cho, S.Y.; Rowe, S.P.; Jain, S.K.; Schon, L.C.; Yung, R.C.; Nayfeh, T.A.; Bingham, C.O., III; Foss, C.A.; Nimmagadda, S.; Pomper, M.G. Evaluation of musculoskeletal and pulmonary bacterial infections with 124I FIAU PET/CT. Mol. Imaging 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Welling, M.M.; Duszenko, N.; van Willigen, D.M.; Smits, W.K.; Buckle, T.; Roestenberg, M.; van Leeuwen, F.W.B. Cyclodextrin/adamantane-mediated targeting of inoculated bacteria in mice. Bioconjug. Chem. 2021. [Google Scholar] [CrossRef]
- Teiler, J.; Ahl, M.; Akerlund, B.; Wird, S.; Brismar, H.; Bjareback, A.; Hedlund, H.; Holstensson, M.; Axelsson, R. Is 99mTc-HMPAO-leukocyte imaging an accurate method in evaluating therapy result in prosthetic joint infection and diagnosing suspected chronic prosthetic joint infection? Q. J. Nucl. Med. Mol. Imaging 2020, 64, 85–95. [Google Scholar] [CrossRef]
- Ebenhan, T.; Zeevaart, J.R.; Venter, J.D.; Govender, T.; Kruger, G.H.; Jarvis, N.V.; Sathekge, M.M. Preclinical Evaluation of 68Ga-labeled 1,4,7-triazacyclononane-1,4,7-triacetic acid-ubiquicidin as a radioligand for PET infection imaging. J. Nucl. Med. 2014, 55, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Silindir-Gunay, M.; Ozer, A.Y. 99mTc-radiolabeled levofloxacin and micelles as infection and inflammation imaging agents. J. Drug Deliv. Sci. Technol. 2020, 56, 101539. [Google Scholar] [CrossRef]
- Shahzadi, S.K.; Qadir, M.A.; Shahzad, S.; Javed, M. 99mTc-amoxicillin: A novel radiopharmaceutical for infection imaging. Arab. J. Chem. 2019, 12, 2533–2539. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, S.; Qadir, M.A.; Rasheed, R.; Ahmed, M. Synthesis of 99mTc-gemifloxacin freeze dried kits and their biodistribution in Salmonella typhi, Pseudomonas aeruginosa and Klebsiella pneumoniae. Arab. J. Chem. 2019, 12, 664–670. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.A.; Jiang, Y.; Gan, Q.; Ruan, Q.; Xiao, D.; Zhang, J. Design, preparation, and evaluation of a novel 99mTcN complex of ciprofloxacin xanthate as a potential bacterial infection imaging agent. Molecules 2020, 25, 5837. [Google Scholar] [CrossRef]
- Welling, M.M.; Lupetti, A.; Balter, H.S.; Lanzzeri, S.; Souto, B.; Rey, A.M.; Savio, E.O.; Paulusma-Annema, A.; Pauwels, E.K.J.; Nibbering, P.H. 99mTc-labeled antimicrobial peptides for detection of bacterial and Candida albicans infections. J. Nucl. Med. 2001, 42, 788–794. [Google Scholar] [PubMed]
- Mustaev, A.; Malik, M.; Zhao, X.; Kurepina, N.; Luan, G.; Oppegard, L.M.; Hiasa, H.; Marks, K.R.; Kerns, R.J.; Berger, J.M.; et al. Fluoroquinolone-gyrase-DNA complexes two modes of drug binding. J. Biol. Chem. 2014, 289, 12300–12312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zhang, W.; Wang, Y.; Jin, Z.; Wang, X.; Zhang, J.; Zhang, Y. Synthesis and biodistribution of a novel 99mTcN complex of norfloxacin dithiocarbamate as a potential agent for bacterial infection imaging. Bioconjug. Chem. 2011, 22, 369–375. [Google Scholar] [CrossRef]
- Abrams, M.J.; Davison, A.; Jones, A.G.; Costello, C.E.; Pang, H. Synthesis and characterization of hexakis (alkyl isocynide) and hexakis (aryl isocyanide) complexes of technetium(I). Inorg. Chem. 1983, 22, 2798–2800. [Google Scholar] [CrossRef]
- Mizuno, Y.; Uehara, T.; Hanaoka, H.; Endo, Y.; Jen, C.-W.; Arano, Y. Purification-free method for preparing technetium-99m-labeled multivalent probes for enhanced in vivo imaging of saturable systems. J. Med. Chem. 2016, 59, 3331–3339. [Google Scholar] [CrossRef]
- Welling, M.M.; Paulusma-Annema, A.; Balter, H.S.; Pauwels, E.K.J.; Nibbering, P.H. Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur. J. Nucl. Med. 2000, 27, 292–301. [Google Scholar] [CrossRef]
- Zhang, X.; Ruan, Q.; Duan, X.; Gan, Q.; Song, X.; Fang, S.; Lin, X.; Du, J.; Zhang, J. Novel 99mTc-labeled glucose derivative for single photon emission computed Tomography: A promising tumor imaging agent. Mol. Pharm. 2018, 15, 3417–3424. [Google Scholar] [CrossRef]
- Van den Wyngaert, T.; Elvas, F.; De Schepper, S.; Kennedy, J.A.; Israel, O. SPECT/CT: Standing on the shoulders of giants, it is time to reach for the sky. J. Nucl. Med. 2020, 61, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Duatti, A. Review on 99mTc radiopharmaceuticals with emphasis on new advancements. Nucl. Med. Biol. 2021, 92, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Taillefer, R.; Primeau, M.; Costi, P.; Lambert, R.; Leveille, J.; Latour, Y. Technetium-99m-sestamibi myocardial perfusion imaging in detection of coronary-artery disease-compaision between intial (1-h) and delayed (3-h) postexercise images. J. Nucl. Med. 1991, 32, 1961–1965. [Google Scholar] [PubMed]






| [99mTc]Tc-CN4NF | [99mTc]Tc-CN5NF | |||||||
|---|---|---|---|---|---|---|---|---|
| Mice with Bacterial Infection | Mice with Turpentine-Induced Abscess | Mice with Bacterial Infection | Mice with Turpentine-Induced Abscess | |||||
| Tissue | 0.5 h | 2 h | 4 h | 4 h | 0.5 h | 2 h | 4 h | 4 h |
| Heart | 1.90 ± 0.23 | 0.74 ± 0.15 | 0.75 ± 0.12 | 1.05 ± 0.06 | 1.77 ± 0.35 | 0.98 ± 0.16 | 0.87 ± 0.10 | 1.12 ± 0.44 |
| Liver | 12.55 ± 1.08 | 14.02 ± 1.04 | 14.18 ± 0.39 | 14.21 ± 1.26 | 14.13 ± 1.43 | 15.51 ± 0.88 | 16.23 ± 1.17 | 16.63 ± 0.53 |
| Lung | 4.99 ± 0.28 | 1.76 ± 0.15 | 1.46 ± 0.03 | 1.61 ± 0.37 | 3.59 ± 0.60 | 1.75 ± 0.49 | 0.93 ± 0.08 | 1.26 ± 0.70 |
| Kidneys | 6.53 ± 1.15 | 5.12 ± 0.61 | 6.00 ± 0.24 | 5.42 ± 0.71 | 5.71 ± 0.80 | 4.28 ± 0.24 | 3.87 ± 0.07 | 2.97 ± 1.03 |
| Spleen | 5.97 ± 1.12 | 7.11 ± 1.44 | 5.11 ± 0.82 | 6.79 ± 2.05 | 4.89 ± 0.83 | 3.80 ± 0.66 | 2.98 ± 0.75 | 3.46 ± 0.83 |
| Stomach | 1.59 ± 0.14 | 1.60 ± 0.26 | 1.37 ± 0.51 | 1.01 ± 0.17 | 5.02 ± 0.82 | 4.53 ± 0.75 | 4.32 ± 0.88 | 1.15 ± 0.24 |
| Bone | 12.08 ± 1.43 | 12.31 ± 1.05 | 14.13 ± 1.10 | 12.20 ± 2.23 | 8.18 ± 0.91 | 9.33 ± 1.45 | 8.25 ± 0.20 | 8.91 ± 1.10 |
| Muscle | 2.71 ± 0.37 | 2.12 ± 0.65 | 1.51 ± 0.46 | 1.10 ± 0.30 | 1.43 ± 0.16 | 0.98 ± 0.14 | 0.56 ± 0.23 | 0.73 ± 0.08 |
| Intestine | 1.41 ± 0.11 | 0.80 ± 0.16 | 0.73 ± 0.18 | 0.81 ± 0.16 | 1.52 ± 0.18 | 0.64 ± 0.22 | 0.68 ± 0.22 | 0.44 ± 0.16 |
| Abscess | 1.89 ± 0.73 | 1.15 ± 0.27 | 0.87 ± 0.06 | 0.53 ± 0.03 | 1.94 ± 0.19 | 1.47 ± 0.08 | 1.17 ± 0.05 | 0.69 ± 0.37 |
| Blood | 2.17 ± 0.38 | 0.74 ± 0.11 | 0.58 ± 0.20 | 0.47 ± 0.08 | 1.87 ± 0.23 | 0.74 ± 0.07 | 0.45 ± 0.03 | 0.45 ± 0.10 |
| Thyroid (%ID) | 0.10 ± 0.02 | 0.10 ± 0.03 | 0.08 ± 0.02 | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.00 |
| T/N 1 | 0.70 | 0.54 | 0.58 | 0.48 | 1.36 | 1.50 | 2.08 | 0.95 |
| T/B 2 | 0.87 | 1.55 | 1.50 | 1.12 | 1.04 | 1.98 | 2.62 | 1.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, S.; Jiang, Y.; Xiao, D.; Zhang, X.; Gan, Q.; Ruan, Q.; Zhang, J. Synthesis and Evaluation of Novel Norfloxacin Isonitrile 99mTc Complexes as Potential Bacterial Infection Imaging Agents. Pharmaceutics 2021, 13, 518. https://doi.org/10.3390/pharmaceutics13040518
Fang S, Jiang Y, Xiao D, Zhang X, Gan Q, Ruan Q, Zhang J. Synthesis and Evaluation of Novel Norfloxacin Isonitrile 99mTc Complexes as Potential Bacterial Infection Imaging Agents. Pharmaceutics. 2021; 13(4):518. https://doi.org/10.3390/pharmaceutics13040518
Chicago/Turabian StyleFang, Si’an, Yuhao Jiang, Di Xiao, Xuran Zhang, Qianqian Gan, Qing Ruan, and Junbo Zhang. 2021. "Synthesis and Evaluation of Novel Norfloxacin Isonitrile 99mTc Complexes as Potential Bacterial Infection Imaging Agents" Pharmaceutics 13, no. 4: 518. https://doi.org/10.3390/pharmaceutics13040518