Film-Forming Systems for the Delivery of DNDI-0690 to Treat Cutaneous Leishmaniasis
Abstract
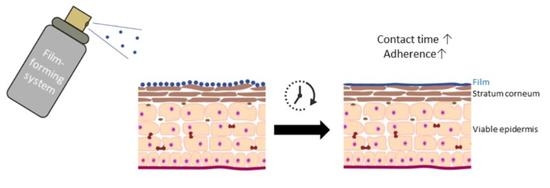
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Materials
2.3. Preparation of the Polymeric Solutions
2.4. Evaluation of the Formulations
2.5. Parasites and Mice
2.6. Permeation Evaluation Using Model Permeant Hydrocortisone
2.7. Skin Permeation Evaluation Using DNDI-0690
2.8. DNDI-0690 Skin Drug Distribution
2.9. DNDI-0690 Quantification by UHPLC-TOF
2.10. In Vivo Efficacy Evaluation
3. Results
3.1. Formulation and Film Evaluation
3.2. In Vitro Hydrocortisone Permeation across the Strat-M Membrane
3.3. DNDI-0690 Release and Distribution in Leishmania-Infected and Uninfected Mouse Skin
3.4. Efficacy of DNDI-0690 Film-Forming Systems against Experimental Cutaneous Leishmaniasis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Velez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Choi, H.L.; Jain, S.; Ruiz Postigo, J.A.; Borisch, B.; Dagne, D.A. The global procurement landscape of leishmaniasis medicines. PLoS Negl. Trop. Dis. 2021, 15, e0009181. [Google Scholar] [CrossRef]
- Alvar, J.; Arana, B. Leishmaniasis—Impact and therapeutic needs. In Drug Discovery for Leishmaniasis; Rivas, L., Gil, C., Eds.; The Royal Society of Chemistry: Croyden, UK, 2018; pp. 3–23. [Google Scholar]
- Bailey, F.; Mondragon-Shem, K.; Haines, L.R.; Olabi, A.; Alorfi, A.; Ruiz-Postigo, J.A.; Alvar, J.; Hotez, P.; Adams, E.R.; Vélez, I.D.; et al. Cutaneous leishmaniasis and co-morbid major depressive disorder: A systematic review with burden estimates. PLoS Negl. Trop. Dis. 2019, 13, e0007092. [Google Scholar] [CrossRef]
- Pires, M.; Wright, B.; Kaye, P.M.; da Conceição, V.; Churchill, R.C. The impact of leishmaniasis on mental health and psychosocial well-being: A systematic review. PLoS ONE 2019, 14, e0223313. [Google Scholar] [CrossRef] [Green Version]
- DNDi Visceral Leishmaniasis: Target Product Profile. Available online: https://dndi.org/diseases/visceral-leishmaniasis/target-product-profile/ (accessed on 2 February 2021).
- DNDi Cutaneous Leishmaniasis: Target Product Profile. Available online: https://dndi.org/diseases/cutaneous-leishmaniasis/target-product-profile/ (accessed on 2 February 2021).
- Frederiksen, K.; Guy, R.H.; Petersson, K. Formulation considerations in the design of topical, polymeric film-forming systems for sustained drug delivery to the skin. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. 2015, 91, 9–15. [Google Scholar] [CrossRef] [Green Version]
- El-On, J.; Halevy, S.; Grunwald, M.H.; Weinrauch, L. Topical treatment of Old World cutaneous leishmaniasis caused by Leishmania major: A double-blind control study. J. Am. Acad. Dermatol. 1992, 27, 227–231. [Google Scholar] [CrossRef]
- Soto, J.; Fuya, P.; Herrera, R.; Berman, J. Topical paromomycin/methylbenzethonium chloride plus parenteral meglumine antimonate as treatment for American cutaneous leishmaniasis: Controlled study. Clin. Infect. Dis. 1998, 26, 56–58. [Google Scholar] [CrossRef] [Green Version]
- Neva, F.A.; Ponce, C.; Ponce, E.; Kreutzer, R.; Modabber, F.; Olliaro, P. Non-ulcerative cutaneous leishmaniasis in Honduras fails to respond to topical paromomycin. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 473–475. [Google Scholar] [CrossRef]
- Ben Salah, A.; Ben Messaoud, N.; Guedri, E.; Zaatour, A.; Ben Alaya, N.; Bettaieb, J.; Gharbi, A.; Belhadj Hamida, N.; Boukthir, A.; Chlif, S.; et al. Topical paromomycin with or without gentamicin for cutaneous leishmaniasis. N. Engl. J. Med. 2013, 368, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Sosa, N.; Pascale, J.M.; Jiménez, A.I.; Norwood, J.A.; Kreishman-Detrick, M.; Weina, P.J.; Lawrence, K.; McCarthy, W.F.; Adams, R.C.; Scott, C.; et al. Topical paromomycin for New World cutaneous leishmaniasis. PLoS Negl. Trop. Dis. 2019, 13, e0007253. [Google Scholar] [CrossRef] [Green Version]
- Couteau, C.; Demé, A.; Cheignon, C.; Coiffard, L.J.M. Influence of the hydrophilic–lipophilic balance of sunscreen emulsions on their water resistance property. Drug Dev. Ind. Pharm. 2012, 38, 1405–1407. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Cross, S.E.; Anissimov, Y.G. Factors affecting the formation of a skin reservoir for topically applied. Skin Pharmacol. Physiol. 2004, 17, 3–16. [Google Scholar] [CrossRef]
- Devaux, S.; Castela, A.; Archier, E.; Gallini, A.; Joly, P.; Misery, L.; Aractingi, S.; Aubin, F.; Bachelez, H.; Cribier, B.; et al. Adherence to topical treatment in psoriasis: A systematic literature review. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 61–67. [Google Scholar] [CrossRef]
- Grada, A.; Feldman, S.R.; Bragazzi, N.L.; Damiani, G. Patient-reported outcomes of topical therapies in actinic keratosis: A systematic review. Dermatol. Ther. 2021, 34, e14833. [Google Scholar]
- Kienzler, J.L.; Queille-Roussel, C.; Mugglestone, C.; Ortonne, J.P.; Larnier, C. Stratum corneum pharmacokinetics of the anti-fungal drug, terbinafine, in a novel topical formulation, for single-dose application in dermatophytoses. Curr. Med. Res. Opin. 2007, 23, 1293–1302. [Google Scholar] [CrossRef]
- Bhutani, T.; Koo, J.; Maibach, H.I. Efficacy of clobetasol spray: Factors beyond patient compliance. J. Dermatol. Treat. 2012, 23, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Van Bocxlaer, K.; Caridha, D.; Black, C.; Vesely, B.; Leed, S.; Sciotti, R.J.; Wijnant, G.J.; Yardley, V.; Braillard, S.; Mowbray, C.E.; et al. Novel benzoxaborole, nitroimidazole and aminopyrazoles with activity against experimental cutaneous leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Fairlamb, A.H.; Patterson, S. Current and Future Prospects of Nitro-compounds as Drugs for Trypanosomiasis and Leishmaniasis. Curr. Med. Chem. 2018, 26, 4454–4475. [Google Scholar]
- Wijnant, G.-J.; Croft, S.L.; de la Flor, R.; Alavijeh, M.; Yardley, V.; Braillard, S.; Mowbray, C.; Van Bocxlaer, K. Pharmacokinetics and Pharmacodynamics of the Nitroimidazole DNDI-0690 in Mouse Models of Cutaneous Leishmaniasis. Antimicrob. Agents Chemother. 2019, 63, e00829-19. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, I.; Franke, P.; Schaefer, U.; Lehr, C. Development and characterization of film forming polymeric solutions for skin. Eur. J. Pharm. Biopharm. 2007, 65, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- Ashland Global Specialty Chemicals Inc. Klucel™ hydroxypropylcellulose—Physical and Chemical Properties. 2021. Available online: www.ashland.com/Documents/PC_11229_Klucel_HPC (accessed on 8 February 2021).
- Wagner, K.G.; Maus, M.; Kornherr, A.; Zifferer, G. Glass transition temperature of a cationic polymethacrylate dependent on the plasticizer content—Simulation vs. experiment. Chem. Phys. Lett. 2005, 406, 90–94. [Google Scholar] [CrossRef]
- Kucera, S.; Shah, N.H.; Malick, A.W.; Infeld, M.H.; McGinity, J.W. Influence of an acrylic polymer blend on the physical stability of film-coated theophylline pellets. Aaps Pharmscitech 2009, 10, 864–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurdo Schroeder, I.; Franke, P.; Schaefer, U.F.; Lehr, C.M. Delivery of ethinylestradiol from film forming polymeric solutions across human epidermis in vitro and in vivo in pigs. J. Control. Release Off. J. Control. Release Soc. 2007, 118, 196–203. [Google Scholar] [CrossRef]
- Frederiksen, K.; Guy, R.H.; Petersson, K. The potential of polymeric film-forming systems as sustained delivery platforms for topical drugs. Expert Opin. Drug Deliv. 2016, 13, 349–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, I.G.; Loria-Kanza, Y.; Jones, T.C. Short-duration topical treatment of tinea pedis using terbinafine emulsion gel: Results of a dose-ranging clinical trial. J. Dermatolog. Treat. 2007, 18, 163–168. [Google Scholar] [CrossRef]
- Van Bocxlaer, K.; Croft, S.L. Pharmacokinetics and pharmacodynamics in the treatment of cutaneous leishmaniasis–Challenges and opportunities. RSC Med. Chem. 2021. [Google Scholar] [CrossRef]
- Van Bocxlaer, K.; Yardley, V.; Murdan, S.; Croft, S.L. Drug permeation and barrier damage in Leishmania-infected mouse skin. J. Antimicrob. Chemother. 2016, 71, 1578–1585. [Google Scholar] [CrossRef] [Green Version]
- Wijnant, G.J.; Van Bocxlaer, K.; Fortes Francisco, A.; Yardley, V.; Harris, A.; Alavijeh, M.; Murdan, S.; Croft, S.L. Local Skin Inflammation in Cutaneous Leishmaniasis as a Source of Variable Pharmacokinetics and Therapeutic Efficacy of Liposomal Amphotericin B. Antimicrob. Agents Chemother. 2018, 62, e00631-18. [Google Scholar] [CrossRef] [Green Version]
- Garvie-Cook, H.; Frederiksen, K.; Petersson, K.; Guy, R.H.; Gordeev, S.N. Biophysical elucidation of the mechanism of enhanced drug release and topical delivery from polymeric film-forming systems. J. Control. Release 2015, 212, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Film-Forming System | Polymer | Plasticiser | EtOH | H2O | |||
|---|---|---|---|---|---|---|---|
| % w/v | Weight (g) | Weight (mg) | Weight (g) | % | Weight (g) | % | |
| Kollidon 90F | 7.5 | 0.375 | 4.625 | 92.5 | |||
| With plasticiser (PG/DBS/TBC/TEC) | 7.5 | 0.375 | 75 | 4.55 | 91 | ||
| Kollidon VA64 | 10 | 0.5 | 4.5 | 90 | |||
| With plasticiser (PG/DBS/TBC/TEC) | 10 | 0.5 | 100 | 4.4 | 88 | ||
| Kollicoat SR 30D | 15 | 0.75 | 4.25 | 85 | |||
| With plasticiser (PG/DBS/TBC/TEC) | 15 | 0.75 | 150 | 4.1 | 82 | ||
| Pemulen TR-2 NF | 2 | 0.1 | 4.9 | 98 | |||
| With plasticiser (PG/DBS/TBC/TEC) | 2 | 0.1 | 20 | 4.88 | 97.6 | ||
| Eudragit RS PO | 10 | 0.5 | 4.25 | 85 | 0.250 | 5 | |
| With plasticiser (PG/DBS/TBC/TEC) | 10 | 0.5 | 100 | 4.15 | 83 | 0.250 | 5 |
| Eudragit NM30 D | 7.5 | 0.375 | 4.125 | 82.5 | 0.5 | 10 | |
| With plasticiser (PG/DBS/TBC/TEC) | 7.5 | 0.375 | 75 | 4.05 | 81 | 0.5 | 10 |
| Klucel LF | 5 | 0.25 | 4.75 | 95 | |||
| With plasticiser (PG/DBS/TBC/TEC) | 5 | 0.25 | 50 | 4.7 | 94 | ||
| Polymers and Plasticiser | Appearance | Viscosity | Drying Time | Stickiness | Cosmetic Attributes | Film Flexibility | Film Removal | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Clear/Opaque | Water (1) Glycerol (2) Lamisil (3) | (Min) | + (Low) ++ (Medium) +++ (High) | Clear/Opaque | Vehicle Drift - (Absent) + (Present) | Cracking - (Absent) + (Low) ++ (Medium) +++ (High) | With Water + (Easy) ++ (Medium) +++ (Difficult) | Peeling - (Absent) + (Present) | ||
| 10% Kollidon VA64 | None | clear | 1 | <2 min | + | clear | - | + | + | - |
| PG | clear | 1 | <2 min | + | clear | - | - | + | - | |
| DBS | clear | 1 | <2 min | + | clear | - | + | + | - | |
| TBC | clear | 1 | <2 min | + | clear | - | - | + | - | |
| TEC | clear | 1 | <2 min | + | clear | - | - | + | - | |
| 10% Eudragit RS PO | None | clear | 1 | <2 min | + | clear | + | ++ | +++ | - |
| PG | clear | 1 | <2 min | + | clear | + | + | +++ | - | |
| DBS | clear | 1 | <2 min | + | clear | + | - | +++ | - | |
| TBC | clear | 1 | <2 min | + | clear | - | - | +++ | - | |
| TEC | clear | 1 | <2 min | + | clear | - | - | ++ | + | |
| 5% Klucel LF Pharm | None | clear | 1–2 | 2–3 min | + | clear | - | - | + | - |
| PG | clear | 1–2 | 3–4 min | + | clear | - | - | + | - | |
| DBS | clear | 1–2 | 3–4 min | + | clear | - | - | + | - | |
| TBC | clear | 1–2 | 3–4 min | + | clear | - | - | + | - | |
| TEC | clear | 1–2 | 2–3 min | + | clear | - | - | + | - | |
| Formulation | Flux (×103 ng/cm2/h) | Lag Time (h) | Kp (cm/h) | |
|---|---|---|---|---|
| Eudragit | Alone | 88 ± 36 | 0.08 | 8.81 × 10−6 |
| DBS | 33 ± 26 | 0.84 | 3.35 × 10−6 | |
| TEC | 131±36 | 0.07 | 16.0 × 10−6 | |
| Klucel | Alone | 196 ± 54 | 1.03 | 19.6 × 10−6 |
| DBS | 254 ± 35 | 0.63 | 25.4 × 10−6 | |
| TEC | 279 ± 92 | 0.67 | 27.9 × 10−6 | |
| Kollidon | Alone | 92 ± 14 | 0 | 9.15 × 10−6 |
| DBS | 117 ± 67 | 0 | 11.7 × 10−6 | |
| TEC | 113 ± 18 | 1.19 | 11.3 × 10−6 | |
| PG–EtOH | 114 ± 60 | 0.69 | 11.4 × 10−6 | |
| EtOH | 140 ± 30 | 1.17 | 14.0 × 10−6 | |
| Formulation | Skin Status | Cumulative Amount/Surface (ng/cm2) | Flux (ng/cm2/h) | Lag Time (h) | Kp (cm/h) |
|---|---|---|---|---|---|
| Eudragit–DBS | Infected | 360 ± 58 | 7.83 ± 1.22 * | 0.14 | 78.3 × 10−6 |
| Uninfected | 212 ± 62 | 5.07 ± 1.44 * | 7.91 | 50.7 × 10−6 | |
| Klucel–TEC | Infected | 212 ± 47 | 4.86 ± 0.92 ** | 8.47 | 48.6 × 10−6 |
| Uninfected | 72 ± 18 | 2.44 ± 0.37 ** | 23.20 | 24.4 × 10−6 | |
| PG–EtOH | Infected | 642 ± 289 | 14.48 ± 6.81 | 9.78 | 14.5 × 10−6 |
| Uninfected | 522 ± 206 | 11.83 ± 4.73 | 8.89 | 11.8 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Bocxlaer, K.; McArthur, K.-N.; Harris, A.; Alavijeh, M.; Braillard, S.; Mowbray, C.E.; Croft, S.L. Film-Forming Systems for the Delivery of DNDI-0690 to Treat Cutaneous Leishmaniasis. Pharmaceutics 2021, 13, 516. https://doi.org/10.3390/pharmaceutics13040516
Van Bocxlaer K, McArthur K-N, Harris A, Alavijeh M, Braillard S, Mowbray CE, Croft SL. Film-Forming Systems for the Delivery of DNDI-0690 to Treat Cutaneous Leishmaniasis. Pharmaceutics. 2021; 13(4):516. https://doi.org/10.3390/pharmaceutics13040516
Chicago/Turabian StyleVan Bocxlaer, Katrien, Kerri-Nicola McArthur, Andy Harris, Mo Alavijeh, Stéphanie Braillard, Charles E. Mowbray, and Simon L. Croft. 2021. "Film-Forming Systems for the Delivery of DNDI-0690 to Treat Cutaneous Leishmaniasis" Pharmaceutics 13, no. 4: 516. https://doi.org/10.3390/pharmaceutics13040516