Continuous Manufacture and Scale-Up of Theophylline-Nicotinamide Cocrystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hot Melt Extrusion Continuous Manufacturing and Feed Rate Calibration
2.3. Residence Time
2.4. Scanning Electron Microscopy
2.5. Particle Size Distribution
2.6. Differential Scanning Calorimetry (DSC)
2.7. X-ray Powder Diffraction
2.8. In Vitro Dissolution Study
2.9. HPLC Analysis
2.10. Surface Dissolution Imaging
2.11. Stability Studies
3. Results
3.1. Hot Melt Extrusion Continuous Processing
3.2. Residence Time
3.3. Scanning Electron Microscopy
3.4. Particle Size Analysis
3.5. Thermal Analysis
3.6. X-ray Powder Diffraction
3.7. In-Vitro Dissolution
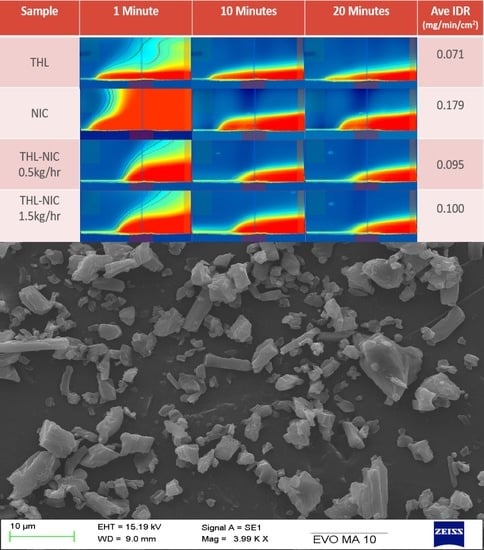
3.8. Surface Dissolution Imaging
3.9. Stability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sastry, S.V.; Nyshadham, J.R.; Fix, J.A. Recent technological advances in oral drug delivery–A review. Pharm. Sci. Technol. Today 2000, 3, 138–145. [Google Scholar] [CrossRef]
- Singh, A.; Worku, Z.A.; Mooter, G.V.D. Oral formulation strategies to improve solubility of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2011, 8, 1361–1378. [Google Scholar] [CrossRef]
- Williams, R.; Watts, A.; Miller, D. Formulating Poorly Water Soluble Drugs; AAPS Press: New York, NY, USA, 2012. [Google Scholar]
- Aitipamula, S.; Banerjee, R.; Bansal, A.; Biradha, K.; Cheney, M.; Choudhury, A.; Desiraju, G.; Dikundwar, A.; Dubey, R.; Duggirala, N.; et al. Polymorphs, salts, and cocrystals: What’s in a name? Crystals Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Schultheiss, N.; Newman, A. Pharmaceutical cocrystals and their physicochemical properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, S.A.; Lamprou, D.A.; Douroumis, D. Engineering and manufacturing of pharmaceutical co-crystals: A review of solvent-free manufacturing technologies. Chem. Commun. 2016, 52, 8772–8786. [Google Scholar] [CrossRef] [Green Version]
- Good, D.J.; Rodríguez-Hornedo, N. Solubility advantage of pharmaceutical cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Steed, J.W. The role of co-crystals in pharmaceutical design. Trends Pharmacol. Sci. 2013, 34, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Ullah, M.; Hussain, I.; Sun, C.C. The development of carbamazepine-succinic acid cocrystal tablet formulations with improved in vitro and in vivo performance. Drug Dev. Ind. Pharm. 2015, 42, 969–976. [Google Scholar] [CrossRef]
- Maheshwari, C.; André, V.; Reddy, S.; Roy, L.; Duarte, T.; Rodríguez-Hornedo, N. Tailoring aqueous solubility of a highly soluble compound via cocrystallization: Effect of coformer ionization, pHmax and solute–solvent interactions. CrystEngComm 2012, 14, 4801–4811. [Google Scholar] [CrossRef]
- Ahmed, H.; Shimpi, M.R.; Velaga, S.P. Relationship between mechanical properties and crystal structure in cocrystals and salt of paracetamol. Drug Dev. Ind. Pharm. 2016, 43, 89–97. [Google Scholar] [CrossRef]
- Holaň, J.; Ridvan, L.; Billot, P.; Štěpánek, F. Design of co-crystallization processes with regard to particle size distribution. Chem. Eng. Sci. 2015, 128, 36–43. [Google Scholar] [CrossRef]
- Rossi, F.; Cerreia Vioglio, P.; Bordignon, S.; Giorgio, V.; Nervi, C.; Priola, E.; Gobetto, R.; Yazawa, K.; Chierotti, M. Unraveling the hydrogen bond network in a theophylline–pyridoxine salt cocrystal by a combined X-ray diffraction, solid-state NMR, and computational approach. Cryst. Growth Des. 2018, 18, 2225–2233. [Google Scholar] [CrossRef]
- Chattoraj, S.; Shi, L.; Sun, C.C. Understanding the relationship between crystal structure, plasticity and compaction behaviour of theophylline, methyl gallate, and their 1: 1 co-crystal. CrystEngComm 2010, 12, 2466–2472. [Google Scholar] [CrossRef]
- Berry, D.J.; Steed, J.W. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, V.; Panicucci, R.; Joshi, Y.; Garad, S. Developability assessment in pharmaceutical industry: An integrated group approach for selecting developable candidates. J. Pharm. Sci. 2009, 98, 1962–1979. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk-Pirek, A. Co-crystal/salt crystal structure disorder of trichloroacetic acid–N-methylurea complex with double system of homo- and heteronuclear O–H··· O/N–H··· O hydrogen bonds: X-ray investigation, ab initio and DFT studies. Struct. Chem. 2012, 23, 1739–1749. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, G.A.; Aburub, A.; Woods, T.A. Physical stability of salts of weak bases in the solid-state. J. Pharm. Sci. 2011, 100, 1607–1617. [Google Scholar] [CrossRef]
- Kuminek, G.; Rodríguez-Hornedo, N.; Siedler, S.; Rocha, H.V.A.; Cuffini, S.L.; Cardoso, S.G. How cocrystals of weakly basic drugs and acidic coformers might modulate solubility and stability. Chem. Commun. 2016, 52, 5832–5835. [Google Scholar] [CrossRef] [Green Version]
- Bolla, G.; Nangia, A. Multicomponent ternary cocrystals of the sulfonamide group with pyridine-amides and lactams. Chem. Commun. 2015, 51, 15578–15581. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Mir, N.A.; Desiraju, G.R. Quaternary cocrystals: Combinatorial synthetic strategies based on long-range synthon Aufbau modules (LSAM). IUCr J. 2016, 3, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Physical stability enhancement of theophylline via cocrystallization. Int. J. Pharm. 2006, 320, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Ende, D.J.A.; Anderson, S.R.; Salan, J.S. Development and scale-up of cocrystals using resonant acoustic mixing. Org. Process. Res. Dev. 2014, 18, 331–341. [Google Scholar] [CrossRef]
- Fucke, K.; Myz, S.A.; Shakhtshneider, T.P.; Boldyreva, E.V.; Griesser, U.J. How good are the crystallisation methods for co-crystals? A comparative study of piroxicam. New J. Chem. 2012, 36, 1969–1977. [Google Scholar] [CrossRef]
- Moradiya, H.G.; Islam, M.T.; Scoutaris, N.; Halsey, S.A.; Chowdhry, B.Z.; Douroumis, D. Continuous manufacturing of high quality pharmaceutical cocrystals integrated with process analytical tools for in-line process control. Cryst. Growth Des. 2016, 16, 3425–3434. [Google Scholar] [CrossRef]
- Narala, S.; Nyavanandi, D.; Srinivasan, P.; Mandati, P.; Bandari, S.; Repka, M.A. Pharmaceutical co-crystals, salts, and co-amorphous systems: A novel opportunity of hot-melt extrusion. J. Drug Deliv. Sci. Technol. 2021, 61, 102209. [Google Scholar] [CrossRef]
- Moradiya, H.G.; Islam, M.T.; Halsey, S.; Maniruzzaman, M.; Chowdhry, B.Z.; Snowden, M.J.; Douroumis, D. Continuous cocrystallisation of carbamazepine and trans-cinnamic acid via melt extrusion processing. CrystEngComm 2014, 16, 3573–3583. [Google Scholar] [CrossRef]
- Fernandes, G.J.; Rathnanand, M.; Kulkarni, V. Mechanochemical synthesis of carvedilol cocrystals utilizing hot melt extrusion technology. J. Pharm. Innov. 2019, 14, 373–381. [Google Scholar] [CrossRef]
- Butreddy, A.; Sarabu, S.; Bandari, S.; Dumpa, N.; Zhang, F.; Repka, M.A. Polymer-assisted aripiprazole–adipic acid cocrystals produced by hot melt extrusion techniques. Cryst. Growth Des. 2020, 20, 4335–4345. [Google Scholar] [CrossRef]
- Daurio, D.; Medina, C.; Saw, R.; Nagapudi, K.; Alvarez-Núñez, F. Application of twin screw extrusion in the manufacture of cocrystals, Part I: Four case studies. Pharmaceutics 2011, 3, 582–600. [Google Scholar] [CrossRef] [Green Version]
- Douroumis, D.; Ross, S.A.; Nokhodchi, A. Advanced methodologies for cocrystal synthesis. Adv. Drug Deliv. Rev. 2017, 117, 178–195. [Google Scholar] [CrossRef]
- Li, P.; Chu, Y.; Wang, L.; Wenslow, R.M.; Yu, K.; Zhang, H.; Deng, Z. Structure determination of the theophylline–nicotinamide cocrystal: A combined powder XRD, 1D solid-state NMR, and theoretical calculation study. CrystEngComm 2014, 16, 3141–3147. [Google Scholar] [CrossRef]
- Mithu, S.H.; Ross, S.A.; Alexander, B.D.; Douroumis, D. Solid state thermomechanical engineering of high-quality pharmaceutical salts via solvent free continuous processing. Green Chem. 2019, 22, 540–549. [Google Scholar] [CrossRef] [Green Version]
- Censi, R.; Gigliobianco, M.R.; Casadidio, C.; Di Martino, P. Hot melt extrusion: Highlighting physicochemical factors to be investigated while designing and optimizing a hot melt extrusion process. Pharmaceutics 2018, 10, 89. [Google Scholar] [CrossRef] [Green Version]
- Ervasti, T.; Aaltonen, J.; Ketolainen, J. Theophylline–nicotinamide cocrystal formation in physical mixture during storage. Int. J. Pharm. 2015, 486, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, R.S.; Kelly, A.L.; York, P.; Coates, P.D.; Paradkar, A.R. Cocrystalization and simultaneous agglomeration using hot melt extrusion. Pharm. Res. 2010, 27, 2725–2733. [Google Scholar] [CrossRef] [PubMed]
- Sarma, B.; Saikia, B. Hydrogen bond synthon competition in the stabilization of theophylline cocrystals. CrystEngComm 2014, 16, 4753–4765. [Google Scholar] [CrossRef]
- Arora, K.K.; Thakral, S.; Suryanarayanan, R. Instability in theophylline and carbamazepine hydrate tablets: Cocrystal formation due to release of lattice water. Pharm. Res. 2013, 30, 1779–1789. [Google Scholar] [CrossRef]
- Aher, S.; Dhumal, R.; Mahadik, K.; Ketolainen, J.; Paradkar, A. Effect of cocrystallization techniques on compressional properties of caffeine/oxalic acid 2:1 cocrystal. Pharm. Dev. Technol. 2011, 18, 55–60. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Topic Q 1 A (R2) Stability Testing of new Drug Substances and Products; EMA: London, UK, 2003; pp. 4–16.
- Lima, A.L.; Pinho, L.A.G.; Chaker, J.A.; Sa-Barreto, L.L.; Marreto, R.N.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Hot-melt extrusion as an advantageous technology to obtain effervescent drug products. Pharmaceutics 2020, 12, 779. [Google Scholar] [CrossRef]
- Douroumis, D. Hot-Melt Extrusion: Pharmaceutical Applications; Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 46–73. [Google Scholar]
- Gao, Y.; Muzzio, F.J.; Ierapetritou, M.G. A review of the Residence Time Distribution (RTD) applications in solid unit operations. Powder Technol. 2012, 228, 416–423. [Google Scholar] [CrossRef]
- Reitz, E.; Podhaisky, H.; Ely, D.; Thommes, M. Residence time modeling of hot melt extrusion processes. Eur. J. Pharm. Biopharm. 2013, 85, 1200–1205. [Google Scholar] [CrossRef]
- Ziegler, G.R.; Aguilar, C.A. Residence time distribution in a co-rotating, twin-screw continuous mixer by the step change method. J. Food Eng. 2003, 59, 161–167. [Google Scholar] [CrossRef]
- Wahl, P.; Hörl, G.; Kaiser, D.; Sacher, S.; Rupp, C.; Shlieout, G.; Breitenbach, J.; Koscher, G.; Khinast, J. In-line measurement of residence time distribution in melt extrusion via video analysis. Polym. Eng. Sci. 2018, 58, 170–179. [Google Scholar] [CrossRef]
- Wesholowski, J.; Berghaus, A.; Thömmes, M. In-line determination of residence time distribution in hot-melt-extrusion. Pharmaceutics 2018, 10, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prat, L.; Guiraud, P.; Rigal, L.; Gourdon, C. Two phase residence time distribution in a modified twin screw extruder. Chem. Eng. Process. Process. Intensif. 1999, 38, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Alhalaweh, A.; Kaialy, W.; Buckton, G.; Gill, H.; Nokhodchi, A.; Velaga, S.P. Theophylline cocrystals prepared by spray drying: Physicochemical properties and aerosolization performance. AAPS PharmSciTech 2013, 14, 265–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koranne, S.; Krzyzaniak, J.F.; Luthra, S.; Arora, K.K.; Suryanarayanan, R. Role of coformer and excipient properties on the solid-state stability of theophylline cocrystals. Cryst. Growth Des. 2019, 19, 868–875. [Google Scholar] [CrossRef]
- Lu, J.; Rohani, S. Preparation and characterization of theophylline−nicotinamide cocrystal. Org. Process. Res. Dev. 2009, 13, 1269–1275. [Google Scholar] [CrossRef]
- Malamatari, M.; Ross, S.A.; Douroumis, D.; Velaga, S.P. Experimental cocrystal screening and solution based scale-up cocrystallization methods. Adv. Drug Deliv. Rev. 2017, 117, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Bruni, G.; Maggi, L.; Mustarelli, P.; Sakaj, M.; Friuli, V.; Ferrara, C.; Berbenni, V.; Girella, A.; Milanese, C.; Marini, A. Enhancing the pharmaceutical behavior of nateglinide by cocrystallization: Physicochemical assessment of cocrystal formation and informed use of differential scanning calorimetry for its quantitative characterization. J. Pharm. Sci. 2019, 108, 1529–1539. [Google Scholar] [CrossRef]
- Padrela, L.; De Azevedo, E.G.; Velaga, S.P. Powder X-ray diffraction method for the quantification of cocrystals in the crystallization mixture. Drug Dev. Ind. Pharm. 2011, 38, 923–929. [Google Scholar] [CrossRef]
- Moradiya, H.; Islam, M.T.; Woollam, G.R.; Slipper, I.J.; Halsey, S.; Snowden, M.J.; Douroumis, D. Continuous cocrystallization for dissolution rate optimization of a poorly water-soluble drug. Cryst. Growth Des. 2014, 14, 189–198. [Google Scholar] [CrossRef]
- Al Rahal, O.; Majumder, M.; Spillman, M.J.; Van De Streek, J.; Shankland, K. Co-crystal structures of furosemide: Urea and carbamazepine: Indomethacin determined from powder X-ray diffraction data. Crystals 2020, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Khamar, D.; Pritchard, R.G.; Bradshaw, I.J.; Hutcheon, G.A.; Seton, L. Polymorphs of anhydrous theophylline: Stable form IV consists of dimer pairs and metastable form I consists of hydrogen-bonded chains. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2011, 67, o496–o499. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Capitelli, F.; Corriero, N.; Cuocci, C.; Falcicchio, A.; Moliterni, A.; Rizzi, R. The Rietveld refinement in the EXPO software: A powerful tool at the end of the elaborate crystal structure solution pathway. Crystals 2018, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Ross, S.A.; Ward, A.; Basford, P.; McAllister, M.; Douroumis, D. Coprocessing of pharmaceutical cocrystals for high quality and enhanced physicochemical stability. Cryst. Growth Des. 2019, 19, 876–888. [Google Scholar] [CrossRef]
- Inam, M.; Wu, J.; Shen, J.; Phan, C.U.; Tang, G.; Hu, X. Preparation and characterization of novel pharmaceutical co-crystals: Ticagrelor with nicotinamide. Crystals 2018, 8, 336. [Google Scholar] [CrossRef] [Green Version]
- Ward, A.; Brown, B.; Walton, K.; Timmins, P.; Conway, B.R.; Asare-Addo, K. Application of focus variation microscopy and dissolution imaging in understanding the behaviour of hydrophilic matrices. Pharmaceutics 2020, 12, 1162. [Google Scholar] [CrossRef]
- Gordon, S.; Naelapää, K.; Rantanen, J.; Selen, A.; Müllertz, A.; Østergaard, J. Real-time dissolution behavior of furosemide in biorelevant media as determined by UV imaging. Pharm. Dev. Technol. 2012, 18, 1407–1416. [Google Scholar] [CrossRef]
- Shevchenko, A.; Bimbo, L.M.; Miroshnyk, I.; Haarala, J.; Jelínková, K.; Syrjänen, K.; Van Veen, B.; Kiesvaara, J.; Santos, H.A.; Yliruusi, J. A new cocrystal and salts of itraconazole: Comparison of solid-state properties, stability and dissolution behavior. Int. J. Pharm. 2012, 436, 403–409. [Google Scholar] [CrossRef]
- Tiwari, R.V.; Patil, H.; Repka, M.A. Contribution of hot-melt extrusion technology to advance drug delivery in the 21st century. Expert Opin. Drug Deliv. 2016, 13, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Agarabi, C.; Zidan, A.S.; Khan, S.R.; Khan, M.A. Physico-mechanical and stability evaluation of carbamazepine cocrystal with nicotinamide. AAPS PharmSciTech 2011, 12, 693–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Zhou, L.; Yang, W.; Li, Y.; Yang, Y.; Zhang, Z.; Wang, C.; Zhang, X.; Yin, Q. Preparation of theophylline-benzoic acid cocrystal and on-line monitoring of cocrystallization process in solution by raman spectroscopy. Crystals 2019, 9, 329. [Google Scholar] [CrossRef] [Green Version]
- Lou, B.; Hu, S. Different hydrogen-bonded interactions in the cocrystals of nicotinamide with two aromatic acids. J. Chem. Crystallogr. 2011, 41, 1663–1668. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Madusanka, N.; Jones, W. Cocrystal dissociation in the presence of water: A general approach for identifying stable cocrystal forms. J. Pharm. Sci. 2014, 103, 2865–2870. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Thakuria, R.; Aldous, B.J.; Jones, W. An investigation of the causes of cocrystal dissociation at high humidity. J. Pharm. Sci. 2014, 103, 2859–2864. [Google Scholar] [CrossRef] [PubMed]











| No | Temperature (Max) (°C) | Screw Speed (rpm) | Throughput (Kg/h) | Cocrystals |
|---|---|---|---|---|
| F1 | 145 | 70 | 0.5 | X |
| F2 | 145 | 100 | 0.5 | X |
| F3 | 165 | 70 | 0.5 | ✓ |
| F4 | 165 | 85 | 0.5 | X |
| F5 | 165 | 70 | 1.5 | X |
| F6 | 165 | 100 | 1.5 | ✓ |
| F7 | 185 | 70 | 0.5 | X |
| F8 | 185 | 100 | 0.5 | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ross, S.A.; Hurt, A.P.; Antonijevic, M.; Bouropoulos, N.; Ward, A.; Basford, P.; McAllister, M.; Douroumis, D. Continuous Manufacture and Scale-Up of Theophylline-Nicotinamide Cocrystals. Pharmaceutics 2021, 13, 419. https://doi.org/10.3390/pharmaceutics13030419
Ross SA, Hurt AP, Antonijevic M, Bouropoulos N, Ward A, Basford P, McAllister M, Douroumis D. Continuous Manufacture and Scale-Up of Theophylline-Nicotinamide Cocrystals. Pharmaceutics. 2021; 13(3):419. https://doi.org/10.3390/pharmaceutics13030419
Chicago/Turabian StyleRoss, Steven A., Andrew P. Hurt, Milan Antonijevic, Nicolaos Bouropoulos, Adam Ward, Pat Basford, Mark McAllister, and Dennis Douroumis. 2021. "Continuous Manufacture and Scale-Up of Theophylline-Nicotinamide Cocrystals" Pharmaceutics 13, no. 3: 419. https://doi.org/10.3390/pharmaceutics13030419