1. Introduction
Buccal mucosa is one of the promising drug administration sites that is becoming attractive for both local and systemic drug delivery [
1,
2]. Buccal mucosa is relatively permeable and has good vascularization [
3,
4]. This route offers many advantages compared to the oral route, such as avoiding gastrointestinal irritation and drug degradation and first-pass liver metabolism, which means it ensures better drug bioavailability [
2,
5,
6]. Moreover, buccal mucosa is accessible, so that a dosage form can be easily administered, leading to better patient compliance compared to other drug dosing routes [
5,
6]. Buccal administration is particularly suitable for the pediatric and geriatric population, as well as patients that have problems with swallowing [
6]. There are many different dosage forms for buccal administration, such as tablets, films, gels, patches, sprays, pastes [
7]. Among them, mucoadhesive buccal films offer several advantages, due to the high flexibility and larger surface area for drug absorption. They ensure more accurate drug dosing compared to gels, which can be easily washed away by the saliva [
2,
7,
8]. Also, in comparison to conventional buccal tablets, films can be more comfortable because they are thin, flexible, and also have good mechanical properties with a resistance to breakage caused by mouth movements [
9,
10]. Mucoadhesive films should have good tensile strength, mucoadhesive properties, and compatibility with the active substance [
11].
Mucoadhesive natural polymers have received significant attention as carriers for buccal films, due to their ability to make close and prolonged contact with mucosa and to optimize drug bioavailability [
10,
12]. In this study, gelatin, the natural bioadhesive polymer, was selected to develop buccal mucoadhesive films. Gelatin is already widely used in pharmaceutical and medical applications and has been recognized as a GRAS (Generally Regarded as Safe) material by the United States Food and Drug Administration [
13]. Gelatin has been used in the pharmaceutical field because of its excellent biodegradability, biocompatibility, non-toxicity, non-immunogenicity, affordability, etc. Gelatin, a natural protein derived from partially denatured collagen, is readily soluble in hot water and forms physically crosslinked hydrogels that are stable below its gelation temperature (≈23 °C) [
14,
15]. Polyelectrolyte complexes readily form between polyanions and polycations. These complex compounds are formed by the ionic association of repeating units on the polymer chains [
16]. The stability of complex compounds is dependent on many environmental factors, such as a solvent’s nature, pH, and ionic strength [
17,
18]. There are two types of gelatin: Type A gelatin (GA), derived from acid-treated processes; and type B gelatin (GB), derived from alkali-treated processes [
19]. Different production processes affect the isoelectric point (pI), pH, and other properties of GA and GB. GA has a pI between 8 and 9 (positive charge at neutral pH), while GB has a pI between 4.8 and 5.4 (negative charge at neutral pH) [
20].
Mucoadhesive strength points to the feasibility of films in terms of their adherence to mucosal membrane. Excessive adhesion may cause harm (irritation) to mucosa, whereas inadequate adhesion will adversely affect therapeutic efficacy, and at the same time, it may cause patient non-compliance. Hence, an optimized mucoadhesion is required for a film to be effectively used as a drug delivery system [
21]. There are four possible general interactions between mucoadhesive polymers and glycoproteins: (1) Covalent attachment; (2) electrostatic interaction, which requires matching of charge groups between the polymer and the mucus; (3) hydrogen bonding; and (4) hydrophobic interactions [
22].
Propranolol hydrochloride (PRH) is a non-selective β-adrenergic blocking agent (β-blocker) and has been used in the treatment of hypertension, arrhythmias, angina pectoris, and many other cardiovascular diseases; moreover, it can be used for migraine prophylaxis, tremor, and anxiety treatment. PRH has low bioavailability after oral administration, due to the extensive first-pass metabolism. This can be a problem because it is reported that approximately only 25% of PRH reaches systemic circulation [
2,
23]. PRH shows pH-dependent solubility; solubility at pH 1.2 is 225 mg/mL, while at pH 6.8 it is 130 mg/mL [
24,
25]. In addition, the drug is stable when the pH level is acidic and decomposes rapidly when it is alkaline. Solutions are most stable at pH 3; in aqueous solutions, propranolol decomposes, due to the oxidation of the isopropylamine side-chain [
25].
In this study, PRH has been chosen as a model drug because it has suitable physicochemical properties (MW 259.34 g/mol, logP = 3.22), it is a BCS class 1 drug (it has high solubility and permeability), and since it undergoes the first-pass metabolism, patients may benefit from buccal administration [
10]. There are many papers that describe the research of PRH in different buccal formulations. Several polymer blends were used. Arbuzzo et al. investigated matrices of chitosan and gelatin for buccal delivery of propranolol hydrochloride. Their group made PRH buccal films [
4] with different ratios of these polymers; buccal tablets with chitosan/gelatin microparticles [
26]; bilayered buccal films based on polyvinylpyrrolidone or polyvinylalcohol with different weight ratios of gelatin or chitosan [
10]. Kraisit et al. developed mucoadhesive buccal film based on hydroxypropyl methylcellulose and polycarbophil loaded with PRH nanoparticles [
27]. Patel et al. prepared buccal films with different ratios of chitosan and polyvinylpyrrolidone K-30 [
28]. Salehi and Boddohi developed mucoadhesive buccal film for co-delivery of rizatriptan benzoate and propranolol hydrochloride using kollicoat
® IR, polyethylene oxide, and hydroxypropyl methylcellulose [
2].
Based on our best knowledge, the comparison of two types of gelatin A and gelatin B as drug carriers for buccal film has not been performed. Aramwit et al. investigated gelatin A and gelatin B nanoparticles as carriers for controlled drug release [
19].
This study aims to comprehensively characterize propranolol hydrochloride/gelatin buccal mucoadhesive films prepared with two types of gelatin: Gelatin from porcine skin, type A (GA), and gelatin from bovine skin (GB). The study was focused on possible drug/carrier interactions and their impact on the mechanical, mucoadhesive, and biopharmaceutical characteristics of the film. Additionally, the bioavailability of the obtained mucoadhesive films was compared with commercially available PRH IR tablets using in silico simulation in GastroPlus™ software. The results from this study will allow researchers the choice of suitable gelatin type to obtain the desired drug release profile.
3. Results and Discussion
3.1. FTIR Analyses
The drug–polymer interaction was checked by comparing the FTIR spectra mixture of the drug containing the GA and GB, with the FTIR spectrum of the pure drug and the pure GA and GB (
Figure 4.).
Pure GA showed the bands at 1662 cm
−1 relative to the vibration of the amide carbonyl group; at 1543 cm
−1 it was associated with stretching of the free amino groups [
4,
15,
57]. The peak at 1243 cm
−1 corresponds to CN stretching, NH in-plane bending, and CH
2 wagging vibrations [
58,
59]. The broad absorption band at 3320 cm
−1 is assigned to the OH stretching and internal hydrogen bonds, which overlap with N−H stretching [
57].
The spectrum of GB showed the bands at 1653 cm
−1 corresponding to the vibration of the amide carbonyl; at 1557 cm
−1 it was associated with the stretching of the free amino groups [
4,
15], 1234 cm
−1 was attributed to combination peaks between C−N stretching vibrations, N−H and the wagging vibrations from CH
2 groups stem from close amino acid residues [
60,
61]. These peaks are known as Amides I, II, and III.
The spectrum of PRH shows peaks at 2965 cm
−1, due to the presence of a secondary amine group; 3283 cm
−1 is due to the hydroxyl group (secondary), the C−O−C stretching in aryl alkyl ether display a stretching band at 1108 cm
−1, and the peak at 797 cm
−1, due to a-substituted naphthalene [
2,
24,
62]. Frequencies of the functional groups of the pure drug remained intact in the mixture with GA (GAP); hence, there was no major interaction between the drug and the GA.
In this part, the electrolytic nature of gelatin and PRH could be considered. Their electrically charged ions could interact in solution in direction to form polyelectrolytic complex (PEC), and Coulomb forces are responsible for this behavior.
The FTIR spectrum of the complex in comparison with the physical mixture is shown in the spectrum for PRH with the GB (GBP). The characteristic carbonyl absorption in 1596 cm
−1 is replaced by the band in the 1550–1560 cm
−1 region. This band corresponds to the auto-symmetrical vibrations of the COO-structure and is used as a diagnosis for the COO-group [
16,
61]. This ionization leads to the possible formation of anion-cation interaction. According to the literature, the formation of PEC should be confirmed with FTIR, DSC, and drug release profile [
4,
8,
16,
56,
63,
64].
FTIR analysis revealed that in GAP, there is no interaction between PRH and GA, while for GBP some results indicate ion-ion reactions. This should be confirmed after DSC analyses and dissolution test.
3.2. DSC Analyses
The results of the DSC analysis of processed series and powders of Gelatin A and Gelatin B (as received) are presented in
Figure 5 and
Table 2. The curve of the pure PRH shows the melting point at 163 °C [
4,
10]. The curves for powders Gelatin A and Gelatin B show the points of transition temperatures at 30.41 °C and 32.35 °C, respectively. Also, there are endothermic peaks for Gelatin A and Gelatin B powders at 121.06 °C and 115.82 °C, respectively. For pure GA film, the points of transition temperature at 44.52 °C and endothermic peak at 85.75 °C were observed [
65]. The glass-to rubber transition associated with polymer molecules started to move and vibrate, and viscoelastic behavior instead of the solid body ensued [
66,
67,
68]. The endothermic peak is associated with the melting of the triple-helix crystalline structure. This peak is followed by processes, such as water evaporation, melting, and recrystallization, of small and/or imperfect gelatin crystallites. Also, because gelatin consists of peptides and proteins produced by partial hydrolysis of collagen, this peak could be obtained, due to overlapping of the glass transition of α-amino acid blocks in the polypeptide chain [
68,
69,
70,
71,
72]. The appearance of this peak is depended on the film preparation conditions (drying). For GB film, those relevant temperatures associated with T
g and endothermic peak were 48.79 °C and 87.89 °C. With the addition of PRH, these temperatures were raised to 53.11 °C and 86.09 °C for GAP and for GBP 60.09 °C and 94.26 °C. There was also the third transition with peak temperature associated with the isomerization of the peptide bonds in gelatin from the trans to the cis configuration and marked as Ti [
73,
74,
75]. For GA and GB films, the values of Ti were 163.33 °C and 177 °C, respectively. It is also found that Ti is correlated with the moisture of gelatin film, and raised when moisture decreased [
75,
76]. In thermograms for films with PRH, Ti was shifted to lower temperatures, 147.32 °C for GAP and 152.55 °C for GBP. The irregularity and shifting of peaks could be attributed to the presence of acetic acid [
60].
According to FTIR analysis, GAP is a physical mixture, while GAB is a complex compound. The DSC results have not provided clear confirmation of the interaction between gelatin and PRH. The rising of characteristic temperatures could be due to the crosslinking of polymer chains with PRH. [
16,
68]. The higher shift of T
g for GAP, then for GBP (accompanying with FTIR analysis), could lead to distinguish the nature of the interaction between gelatin A or B and PRH.
In both samples with the drug (GAP and GBP), a PRH crystallization peak was not found. The amorphous form was therefore obtained, indicating better solubility and bioavailability for both films [
10].
3.3. SEM Analysis
In
Figure 6, film cross-sections of pure gelatin A and gelatin B and films loaded with PRH are presented. The images show that films are smooth and nonporous. It can also be seen that films have good homogeneity and no presence of PRH powder.
3.4. Mechanical Properties
Tensile curves and the results of the tensile test are presented in
Figure 7 and
Table 3. The mechanical properties of natural polymers are influenced by the preparation process, temperature, and moisture, so it is difficult to compare them with other references. However, it could be seen that the pure GA and GB results are in agreement with some of the data from the literature [
32]. Film GA with a higher bloom index showed higher tensile strength, elastic modulus, and elongation at break, as expected [
32]. For mucoadhesive buccal films, there are no recommended specific values for tensile strength and modulus of elasticity. Results obtained in this study for GAP and GBP are in range with the other published results for mucoadhesive buccal films with different compositions (i.e., hydroxypropyl methylcellulose (HPMC)/polycarbophil (PC) blend, polyvinylalcohol (PVA), and polyvinylpyrrolidone (PVP) blend and more complex composition [
27,
37,
40,
77,
78,
79,
80].
It can be noted that the tensile strength and elastic modulus decreased with the addition of PRH both in GA and GB, while the elongation at break increased by three orders of magnitude. Also, it could be seen from the curves’ shapes that the plasticity of the films with PRH was raised. The addition of drug in gelatin leads to higher free volume and make the easier moving of polymer molecules. In pure gelatin, chains are tangled. During solvent casting, drug molecules were positioned between the polymer chains and increased the possibility of film deformation.
The results of micro-hardness are presented in
Figure 8. Hardness represents the ability of materials to respond to plastic deformation. GA showed a slightly lower hardness than GB. Regardless of the physical mixture in GAP in comparison to the complex in GBP, the hardness of GAP was higher than for GBP. The addition of PRH in GA acted as physical crosslinking of the polymer, while in GBP, a complex was formed [
16,
19].
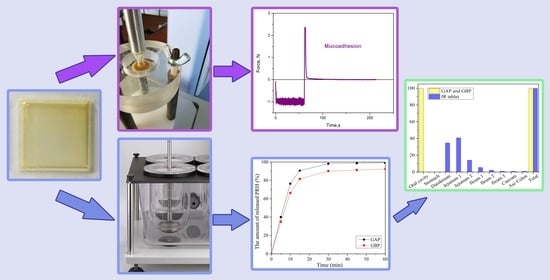
3.5. Mucoadhesion Studies
When two parts of material are connected together via surfaces, the force of their detachment is the measure of their adhesion [
81].
A test for mucoadhesion was performed in accordance with the fracture theory, and the results are presented in
Table 4. In fracture theory, the force required for the detachment of a film is related to the strength of the adhesive bond, while work of adhesion (W
adh) is total energy involved for this separation, and it can be calculated as the area under force–distance curve [
82]. It could be seen that the strength of adhesion (detachment force, F
adh) of the gelatin increases with the addition of PRH. The force of adhesion for pure GB is higher than for pure GA for almost 10%. Strength of adhesion increased with the addition of PRH to the gelatin, both for GAP and GBP in relation to pure GA and GB. The energy or the work of adhesion (W
adh) is associated with crosslinking of polymers; it is higher when the crosslinking is reduced. Polymers with lower crosslinking easily diffuse and entangle with mucin fibers. From
Table 4, it can be observed that adding PRH to GA leads to higher energy. The bonding of mucin and film may be either primary (like covalent bonds) or secondary, like van der Waals bonding, hydrogen bonding, hydrophobic inter-actions, or electrostatic forces. This result should be combined with an electrostatic theory about electro attraction between anionic polymers (GA) with negatively charged sialic acid and sulfate residues of mucin glycoprotein [
10,
76,
81,
82]. For GB, adding PRH leads to a lower value of the work of adhesion. W
adh is the energy associated with surface deformation in the detachment and fracture mode of adhesive material. During detachment of two surfaces occur the crack formation and its propagation. The behavior of GBP in adhesion test shows viscoelasticity, while a brittle detachment fracture occurs. Like in the case of tension, when toughness is determined as the area under stress–strain curve, it is possible to maintain higher force with the lower area under the curve. The reversible work of adhesion could be described as the sum of the components corresponding to molecular interaction, surface deformation, and instabilities in adhesion and subsequent separation [
83,
84]. Moreover, it could be said that the lower W
adh implies a higher crosslinking of the polymer. This state causes a slower intake of fluid into the polymer and decreases the rate of polymer molecules and mucin interpenetration [
22]. As the crosslinking density of the polymer is increased, the diffusion of fluid decreases. The slowdown in diffusion is more severe at the polymer-water interface [
85].
The work of adhesion for GAP is the highest. The presence of a higher number of amino groups leads to a lower density of crosslinking. Due to this fact a much better intake of water into the film occurs, and all of this results in swelling. On the other hand, the highest force of adhesion occurs with GBP, but at the same time, the lowest work of adhesion can be observed. That means that, in this way, the highest density of crosslink is achieved.
Table 5 shows the results of the statistical analysis for force as the dependent variable and
Table 6 for work as the dependent variable.
It can be concluded from the results of the statistical analysis that neither of the factors nor their interaction is statistically significant, as all p values are higher than 0.05.
3.6. Drug Content Uniformity
Drug content uniformity percentage is in the range of 98.61 ± 3.35 for GAP and 99.28 ± 3.74 for GBP. The results showed that there were acceptable changes in drug’s content, so the films have uniform drug distribution.
3.7. In-Vitro Release Study
The release of the drug from the buccal film is determined by drug/polymer properties. It also depends on the drug solubility, the drug diffusion from the film, the swelling, and degradation of the polymer matrix [
42,
57,
86,
87]. Although propranolol hydrochloride solubility is not expected to be the rate-limiting step for its dissolution and absorption [
52], a dissolution study was performed under pH conditions that correspond to the physiological pH in the oral cavity (pH of the deionized water was 6.8). Other properties of the oral cavity fluid, such as ion concentration, osmolality, surface tension, viscosity, etc., have not been considered in this study; however, swelling and dissolution of some natural polymers may depend on these conditions. In this case, the diffusional force is much more important for the drug release than the films’ degradation because of the gelatin concentration of 20% [
57,
87]. Therefore, it is very important to evaluate in vitro release profile of the buccal film [
42].
In vitro release of PRH from GAP and GBP, films are shown in
Figure 9. Drug release happened immediately out of both formulations. More than 50% of PRH was released in the first 10 min and more than 80% in the first 15 min. The release was better from GAP films because the polymer and the drug are a physical mixture in comparison to GBP, where the complex was formed, which was shown on the FTIR spectra and the DSC analysis. There was a complete release of PRH from GAP, and a bit less from GBP because of the complex formation [
4,
10]. Moreover, the density of crosslink has a role in the release of PRH, as was previously mentioned in the mucoadhesion discussion.
Here, we also need to note that under the in vivo conditions, a drug can diffuse through both sides of the film, but since the designed formulations were intended for immediate drug release, as confirmed by our dissolution test results, the applied test conditions can be considered acceptable.
3.8. Physiologically-Based Simulations
Using the input data noted in
Table 1, together with the drug dissolution profiles, shown in
Figure 9, the expected propranolol absorption and disposition pattern has been predicted following the buccal application of the designed films. Here we adjusted the simulation setup (i.e., contact surface area) to comply with the dissolution test conditions, which is justified by the obtained fast drug release profiles, but as implied earlier, the drug diffusion can occur through both sides of the film under in vivo conditions.
The simulated plasma concentration-time profiles are depicted in
Figure 10, and the corresponding pharmacokinetic parameters are given in
Table 7.
The obtained results indicate negligible differences in the rate and extent of propranolol absorption from the tested formulations, with only about a 3% decrease in t
max and a 2% decrease in area under the plasma concentration-time curve (AUC
0–∞) for the formulation GAP in comparison to GBP. These slight differences are caused by a somewhat slower drug dissolution rate from the formulation GBP, but in general, the profiles can be considered similar. The same congruency applies for the simulated regional drug absorption from the tested films, indicating complete absorption from the buccal region (
Figure 11). Such high bioavailability can be considered a substantial benefit of propranolol buccal films [
5,
7,
88,
89,
90]. Namely, in comparison to commercially available oral dosage forms, e.g., 80 mg propranolol IR tablets, the drug absorption is complete for both buccal and oral dosing routes (
Figure 11 and
Table 5), but due to the extensive first-pass metabolism in the liver, the drug bioavailability following oral dosing is notably decreased (
Table 7). Due to the improved propranolol bioavailability from buccal films, the therapeutic drug dosing or dosages can be markedly reduced, e.g., the extent of drug absorption, expressed as AUC, obtained from 30 mg PRH buccal films is in the same range as obtained from 80 mg PRH IR tablet (which is less than 10% difference). Future in vivo studies are encouraged to support our in silico prediction results.
4. Conclusions
In this work, two types of gelatin (gelatin from porcine skin, type A (GA), and gelatin from bovine skin (GB)), were compared as suitable candidates for PRH carriers in the form of mucoadhesive buccal films. A comprehensive characterization was implemented to gain insight into the phenomenology of processing, morphology, mechanical and mucoadhesive behavior, and the drug delivery as well. PRH was chosen for the model drug because in a buccal film, it has a possibility to avoid loss during extensive first-pass metabolism. Elucidation of pure gelatin films structure (GA and GB) and also ones loaded with PRH (GAP and GBP) were investigated and compared.
Both types of drug-loaded films have shown good morphological homogeneity and have contained the amorphous form of PRH, which leads to better bioavailability of the drug. This was also confirmed by in silico simulation of regional absorption profiles, and due to the improved PRH bioavailability from buccal films in comparison with the immediate-release tablets, the therapeutic drug dose can be markedly reduced. The obtained GAP film shows 2.5 times higher elastic modulus, more than four times higher tensile strength, and 33% higher hardness in comparison to GBP film. Statistical analysis of the mucoadhesion tests have shown that neither polymer type nor drug nor their interaction had a statistically significant influence the strength and work of adhesion. Both films, GAP and GBP, have a similar drug release profile, with a slightly slower dissolution rate for GBP. Some tests and analyses were conducted regarding the ionic nature of GA, GB, and the drug. The dissolution test, FTIR, and DSC analysis together indicate that in GBP, the ion-ion complex interaction was more pronounced, while GA formed a physical mixture with PRH. The results presented in this work allowed us to estimate that GAP film will have better processing availability and higher solubility followed by faster drug release. On the other hand, GBP showed slightly lower mechanical strength and slower drug release profile, but stronger mucoadhesion force. In conclusion, gelatin-based mucoadhesive films with PRH have good potential for drug delivery to the buccal mucosa, allowing targeted delivery and reduced drug dosing.