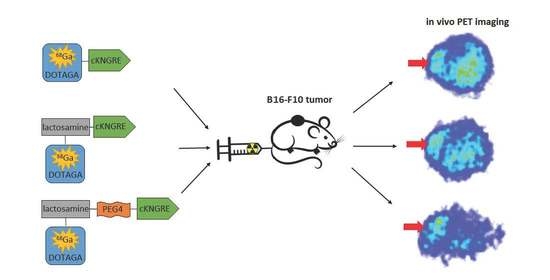
Synthesis of 68Ga-Labeled cNGR-Based Glycopeptides and In Vivo Evaluation by PET Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Chemistry
2.2.1. DOTAGA-LacN (3)
2.2.2. DBCO-cKNGRE (6)
2.2.3. DBCO-PEG4-cKNGRE (8)
2.2.4. DOTAGA-LacN-cKNGRE (9)
2.2.5. DOTAGA-LacN-PEG4-cKNGRE (10)
2.2.6. DOTAGA-cKNGRE (11)
2.3. Radiochemistry
2.3.1. 68Ga Labeling of DOTAGA-LacN (3), DOTAGA-LacN-cKNGRE (9) and DOTAGA-LacN-PEG4-cKNGRE (10)
2.3.2. 68Ga Labeling of DOTAGA-cKNGRE (11)
2.3.3. Determination of logP Value of [68Ga]-3, [68Ga]-9, [68Ga]-10 and [68Ga]-11
2.3.4. Determination of In Vitro Stability of [68Ga]-3, [68Ga]-9, [68Ga]-10 and [68Ga]-11
2.4. Biology
2.4.1. Animal Housing
2.4.2. B16-F10 Tumor Induction
2.4.3. In Vivo PET Imaging and Image Analysis
2.4.4. Statistical Analysis
3. Results
3.1. Chemistry
3.2. Radiochemistry
3.3. Biology
4. Diccussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simons, M. Angiogenesis, where do we stand now? Circulation 2005, 111, 1556–1566. [Google Scholar] [CrossRef] [Green Version]
- Wickstrom, M.; Larsson, R.; Nygren, P.; Gullbo, J. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011, 102, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, R.; Koivunen, E.; Kain, R.; Lahdenranta, J.; Sakamoto, M.; Stryhn, A.; Ashmum, R.A.; Shapiro, L.H.; Arap, W.; Ruoslahti, E. Aminopeptidase N is a Receptor for Tumor-homing peptides and a Target for Inhibiting Angiogenesis. Cancer Res. 2000, 60, 722–727. [Google Scholar]
- Corti, A.; Curnis, F.; Arap, W.; Pasqualini, R. The neovasculature homing motif NGR: More than meets the eye. Blood 2008, 112, 2628–2635. [Google Scholar] [CrossRef] [Green Version]
- Corti, A.; Curnis, F. Tumor vasculature targeting through NGR peptide-based drug delivery systems. Curr. Pharm. Biotechnol. 2011, 12, 1128–1134. [Google Scholar] [CrossRef]
- Dijkgraaf, I.; van de Vijver, P.; Dirksen, A.; Hackeng, T.M. Synthesis and application of cNGR-containing imaging agents for detection of angiogenesis. Bioorg. Med. Chem. 2013, 21, 3555–3564. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ding, Z.; Li, X.; Wei, H.; Chen, Y. Research Progress of Radiolabeled Asn-Gly-Arg (NGR) Peptides for Imaging and Therapy. Mol. Imaging 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Lorusso, D.; Scambia, G.; Amadio, G.; Di Legge, A.; Pietragalla, A.; De Vincenzo, R.; Masciullo, V.; Di Stefano, M.; Mangili, G.; Citterio, G.; et al. Phase II study of NGR-hTNF in combination with doxorubicin in relapsed ovarian cancer patients. Br. J. Cancer 2012, 107, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Parmiani, G.; Pilla, L.; Corti, A.; Doglioni, C.; Cimminiello, C.; Bellone, M.; Parolini, D.; Russo, V.; Capocefalo, F.; Maccalli, C. A pilot phase I study combining peptide-based vaccination and NGR-hTNF vessel targeting therapy in metastatic melanoma. OncoImmunology 2014, 3, e963406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregorc, V.; Gaafar, R.M.; Favaretto, A.; Grossi, F.; Jassem, J.; Polychronis, A.; Bidoli, P.; Tiseo, M.; Shah, R.; Taylor, P.; et al. NGR-hTNF in combination with best investigator choice in previously treated malignant pleural mesothelioma (NGR015): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2018, 19, 799–811. [Google Scholar] [CrossRef]
- Fani, M.; Nicolas, G.P.; Wild, D. Somatostatin Receptor Antagonists for Imaging and Therapy. J. Nucl. Med. 2017, 58, S61–S66. [Google Scholar] [CrossRef]
- Tirosh, A.; Kebebew, E. The utility of 68Ga-DOTATATE positron-emission tomography/computed tomography in the diagnosis, management, follow-up and prognosis of neuroendocrine tumors. Future Oncol. 2018, 14, 111–122. [Google Scholar] [CrossRef]
- Chen, K.; Ma, W.; Li, G.; Wang, J.; Yang, W.; Yap, L.P.; Hughes, L.D.; Park, R.; Conti, P.S. Synthesis and Evaluation of 64Cu-Labeled Monomeric and Dimeric NGR Peptides for MicroPET Imaging of CD13 Receptor Expression. Mol. Pharm. 2013, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Jun, Z.; Xiaoli, L.; Nan, W.; Zichun, H.; Zizheng, W.; Hongbo, H.; Min, Y.; Feng, W. 68Ga-DOTA-NGR as a novel molecular probe for APN-positive tumor imaging using microPET. Nucl. Med. Biol. 2014, 41, 268–275. [Google Scholar]
- Máté, G.; Kertész, I.; Enyedi, N.K.; Mező, G.; Angyal, J.; Vasas, N.; Kis, A.; Szabó, É.; Emri, M.; Bíró, T.; et al. In vivo imaging of amino peptidase N (CD13) receptors in experimental renal tumors using the novel radiotracer 68Ga-NOTA-c(NGR). Eur. J. Pharm. Sci. 2015, 69, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kis, A.; Dénes, N.; Szabó, J.P.; Arató, V.; Jószai, I.; Enyedi, K.N.; Lakatos, S.; Garai, I.; Mező, G.; Kertész, I.; et al. In vivo assessment of aminopeptidase N (APN/CD13) specificity of different 68Ga-labelled NGR derivatives using PET/MRI imaging. Int. J. Pharm. 2020, 589, 119881. [Google Scholar] [CrossRef]
- Mohtavinejad, N.; Ardestani, M.S.; Khalaj, A.; Pormohammad, A.; Najafi, R.; Bitarafan-Rajabi, A.; Hajiramezanali, M.; Amanlou, M. Application of radiolabeled peptides in tumor imaging and therapy. Life Sci. 2020, 258, 118206. [Google Scholar] [CrossRef]
- Gharibkandi, N.A.; Conlon, J.M.; Hosseinimehr, S.J. Strategies for improving stability and pharmacokinetic characteristics of radiolabeled peptides for imaging and therapy. Peptides 2020, 133, 170385. [Google Scholar] [CrossRef]
- Evans, B.J.; King, A.T.; Katsifis, A.; Matesic, L.; Jamie, J.F. Methods to Enhance the Metabolic Stability of Peptide-Based PET Radiopharmaceuticals. Molecules 2020, 25, 2314. [Google Scholar] [CrossRef]
- Seetharaman, J.; Kaningsberg, A.; Slaaby, R.; Leffler, H.; Barondes, S.H.; Rini, J.M. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-Å resolution. J. Biol. Chem. 1998, 273, 13047–13052. [Google Scholar] [CrossRef] [Green Version]
- Ehlerding, E.B.; Sun, L.; Lan, X.; Zeng, D.; Cai, W. Dual-targeted molecular imaging of cancer. J. Nucl. Med. 2018, 59, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Danguy, A.; Camby, I.; Kiss, R. Galectins and cancer. Biochim. Biophys. Acta 2002, 1572, 285–293. [Google Scholar] [CrossRef]
- Comodo, A.N.; Bachi, A.L.L.; Soares, M.F.; Franco, M.F.; Teixeira, V.D. Galectin-3 expression favors metastasis in murine melanoma. Adv. Biosci. Biotechnol. 2013, 4, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Endo, K.; Kohnoe, S.; Tsujita, E.; Watanabe, A.; Nakashima, H.; Baba, H.; Maehara, Y. Galectin-3 expression is a potent prognostic marker in colorectal cancer. Anticancer Res. 2005, 25, 3117–3121. [Google Scholar]
- Arfaoui-Toumi, A.; Mahmoud, L.K.-B.; Hmida, M.B.; Khalfallah, M.T.; Regaya-Mzabi, S.; Bouraoui, S. Implication of the Galectin-3 in colorectal cancer development (about 325 Tunisian patients). Bull. Cancer 2010, 97, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Judmann, B.; Braun, D.; Wängler, B.; Schirrmacher, R.; Fricker, G.; Wängler, C. Current State of Radiolabeled Heterobivalent Peptidic Ligands in Tumor Imaging and Therapy. Pharmaceuticals 2020, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Bajza, I.; Dekany, G.; Agoston, K.; Perez, I.F.; Boutet, J.; Hederos, M.; Horvath, F.; Kovacs-Penzes, P.; Kroeger, L.; Roehrig, C.; et al. A Method for Preparation of the Tetrasaccharide lacto-N-neotetraose (LNnt) Containing N-acetyllactosamine. Patent WO 2011100980 A1, 25 August 2011. [Google Scholar]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.W.; Cummings, R.D.; Drickamer, K.; Felzi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K.I.; et al. Galectins: A family of animal β-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef]
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Wang, Z.; Hao, M.; Li, J. Bispecific antibodies and their applications. J. Hematol. Oncol. 2015, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Gyuricza, B.; Szabó, J.P.; Arató, V.; Szücs, D.; Vágner, A.; Szikra, D.; Fekete, A. Synthesis of Novel, Dual-Targeting 68Ga-NODAGA-LacN-E[c(RGDfK)]2 Glycopeptide as a PET Imaging Agent for Cancer Diagnosis. Pharmaceutics 2021, 13, 796. [Google Scholar] [CrossRef]
- Kis, A.; Dénes, N.; Péli-Szabó, J.; Arató, V.; Beke, L.; Matolay, O.; Enyedi, K.; Méhes, G.; Mező, G.; Bai, P.; et al. In Vivo Molecular Imaging of the Efficacy of Aminopeptidase N (APN/CD13) Receptor Inhibitor Treatment on Experimental Tumors Using 68Ga-NODAGA-c(NGR) Peptide. BioMed Res. Inter. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.G.; Kim, S.J.; Baek, J.H.; Lee, H.W.; Jeong, S.Y.; Chun, K.H. Galectin-3 increases the motility of mouse melanoma cells by regulating matrix metalloproteinase-1 expression. Exp. Mol. Med. 2012, 44, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Barnieh, F.M.; Loadman, P.M.; Falconer, R.A. Is tumour-expressed aminopeptidase N (APN/CD13) structurally and functionally unique? Biochim. Biophys. Acta BBA Rev. Cancer 2021, 1876, 88641. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Kuo, P.L. The Role of Galectin-3 in the Kidneys. Int. J. Mol. Sci. 2016, 17, 565. [Google Scholar] [CrossRef] [Green Version]
- Hsu, D.K.; Dowling, C.A.; Jeng, K.C.; Chen, J.T.; Yang, R.Y.; Liu, F.T. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int. J. Cancer 1999, 81, 519–526. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyuricza, B.; Szabó, J.P.; Arató, V.; Dénes, N.; Szűcs, Á.; Berta, K.; Kis, A.; Szücs, D.; Forgács, V.; Szikra, D.; et al. Synthesis of 68Ga-Labeled cNGR-Based Glycopeptides and In Vivo Evaluation by PET Imaging. Pharmaceutics 2021, 13, 2103. https://doi.org/10.3390/pharmaceutics13122103
Gyuricza B, Szabó JP, Arató V, Dénes N, Szűcs Á, Berta K, Kis A, Szücs D, Forgács V, Szikra D, et al. Synthesis of 68Ga-Labeled cNGR-Based Glycopeptides and In Vivo Evaluation by PET Imaging. Pharmaceutics. 2021; 13(12):2103. https://doi.org/10.3390/pharmaceutics13122103
Chicago/Turabian StyleGyuricza, Barbara, Judit P. Szabó, Viktória Arató, Noémi Dénes, Ágnes Szűcs, Katalin Berta, Adrienn Kis, Dániel Szücs, Viktória Forgács, Dezső Szikra, and et al. 2021. "Synthesis of 68Ga-Labeled cNGR-Based Glycopeptides and In Vivo Evaluation by PET Imaging" Pharmaceutics 13, no. 12: 2103. https://doi.org/10.3390/pharmaceutics13122103