The Use of Menthol in Skin Wound Healing—Anti-Inflammatory Potential, Antioxidant Defense System Stimulation and Increased Epithelialization
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals
2.2. Development of Creams Used in Skin Wound Treatment
2.3. Skin Wound Procedure and Treatment
2.4. Wound Area Contraction Rate
2.5. Histological Analysis
2.6. Analysis of Antioxidant and Myeloperoxidase (MPO) Activities
2.7. Analysis of Anti-Inflammatory Activity
2.8. Analysis by Real-Time Quantitative Gene Expression: RT-qPCR
2.9. Statistical Analysis
3. Results
3.1. Skin Wounds Healing
3.2. Histological Analysis
3.3. MPO and Antioxidant Activities
3.4. Anti-Inflammatory Activity
3.5. Gene Expression
4. Discussion
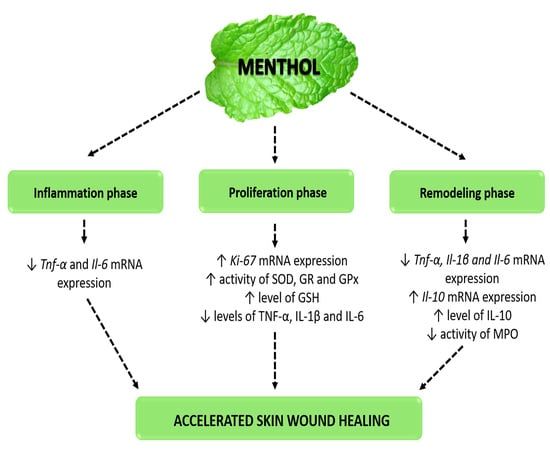
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goswami, S.; Kandhare, A.; Zanwar, A.A.; Hegde, M.V.; Bodhankar, S.L.; Shinde, S.; Deshmukh, S.; Kharat, R. Oral L-glutamine administration attenuated cutaneous wound healing in Wistar rats. Int. Wound J. 2019, 13, 116–124. [Google Scholar] [CrossRef]
- Laberge, A.; Arif, S.; Moulin, V.J. Microvesicles: Intercellular messengers in cutaneous wound healing. J. Cell Physiol. 2018, 233, 5550–5563. [Google Scholar] [CrossRef]
- Profyris, C.; Tziotzios, C.; Do Vale, I. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics Part I. The molecular basis of scar formation. J. Am. Acad. Dermatol. 2012, 66, 1–10. [Google Scholar] [CrossRef]
- Pang, Y.; Zhang, Y.; Huang, L.; Xu, L.; Wang, K.; Wang, D.; Guan, L.; Zhang, Y.; Yu, F.; Chen, Z.; et al. Effects and Mechanisms of Total Flavonoids from Blumea balsamifera (L.) DC. on Skin Wound in Rats. Int. J. Mol. Sci. 2017, 18, 2766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemmati, A.A.; Larki-Harchegani, A.; Shabib, S.; Jalali, A.; Rezaei, A.; Housmand, G. Wound healing property of milk in full thickness wound model of rabbit. Int. J. Surg. 2018, 54, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kandhare, A.D.; Alam, J.; Patil, M.V.; Sinha, A.; Bodhankar, S.L. Wound healing potential of naringin ointment formulation via regulating the expression of inflammatory, apoptotic and growth mediators in experimental rats. Pharm. Biol. 2016, 54, 419–432. [Google Scholar] [CrossRef]
- Zeng, R.; Lin, C.; Lin, Z.; Chen, H.; Lu, W.; Lin, C.; Li, H. Approaches to cutaneous wound healing: Basics and future directions. Cell Tissue Res. 2018, 374, 217–232. [Google Scholar] [CrossRef]
- Öz, B.E.; İşcan, G.S.; Akkol, E.K.; Süntar, İ.; Acıkara, Ö.B. Isofavonoids as wound healing agents from Ononidis radix. J. Ethnopharmacol. 2018, 211, 384–393. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Yaochite, J.N.U.; Sasahara, G.L.; Albuquerque, A.A.; Fonseca, S.G.C.; Araújo, T.D.V.; Santiago, G.M.P.; de Sousa, L.M.; de Carvalho, J.L.; Alves, A.P.N.N.; et al. Antioxidant, anti-inflammatory and healing potential of ethyl acetate fraction of Bauhinia ungulata L. (Fabaceae) on in vitro and in vivo wound model. Mol. Biol. Rep. 2020, 47, 2845–2859. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.R.; Aboud, E. Quercetin and low level laser therapy promote wound healing process in diabetic rats via structural reorganization and modulatory effects on inflammation and oxidative stress. Biomed. Pharm. 2018, 101, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Aruna, S.M.; Sravanthi, V.; Sri, U.J.; Priya, N.S.; Rama, R.N. An overview of herbs possessing wound healing activity. Eur. J. Pharm Med. Res. 2015, 7, 329–332. [Google Scholar]
- Croteau, R.B.; Davis, E.M.; Ringer, K.L.; Wildung, M.R. (−)-Menthol biosynthesis and molecular genetics. Naturwissenschaften 2005, 92, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Limpanuparb, T.; Lorpaiboon, W.; Chinsukserm, K. An in silico investigation of menthol metabolism. PLoS ONE 2019, 14, e0216577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isman, E.; Aras, M.H.; Cengiz, B.; Bayraktar, R.; Yolcu, U.; Topcuoglu, T.; Usumez, A.; Demir, T. Effects of laser irradiation at different wavelengths (660, 810, 980, and 1064 nm) on transient receptor potential melastatin channels in an animal model of wound healing. Lasers Med. Sci. 2015, 30, 1489–1495. [Google Scholar] [CrossRef]
- Nguyen, T.H.D.; Itoh, S.G.; Okumura, H.; Tominaga, M. Structural basis for promiscuous action of monoterpenes on TRP channels. Commun. Biol. 2021, 4, 293. [Google Scholar] [CrossRef]
- Miyamoto, T.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat. Commun. 2011, 2, 369. [Google Scholar] [CrossRef]
- Aijima, R.; Wang, B.; Takao, T.; Mihara, H.; Kashio, M.; Ohsaki, Y.; Zhang, J.Q.; Mizuno, A.; Suzuki, M.; Yamashita, Y.; et al. The thermosensitive TRPV3 channel contributes to rapid wound healing in oral epithelia. FASEB J. 2015, 29, 182–192. [Google Scholar] [CrossRef]
- Yamada, T.; Ueda, T.; Ugawa, S.; Ishida, Y.; Imayasu, M.; Koyama, S.; Shimada, S. Functional expression of transient receptor potential vanilloid 3 (TRPV3) in corneal epithelial cells: Involvement in thermosensation and wound healing. Exp. Eye Res. 2010, 90, 121–129. [Google Scholar] [CrossRef]
- Xu, H.; Blair, N.T.; Clapham, D.E. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J. Neurosci. 2005, 25, 8924–8937. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013, 154, 2169–2177. [Google Scholar] [CrossRef] [Green Version]
- Patel, T.; Ishiuji, Y.; Yosipovitch, G. Menthol: A refreshing look at this ancient compound. J. Am. Acad. Derm. 2007, 57, 873–878. [Google Scholar] [CrossRef]
- Haeseler, G.; Maue, D.; Grosskreutz, J.; Bufler, J.; Nentwig, B.; Piepenbrock, S.; Dengler, R.; Leuwer, M. Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. Eur. J. Anaesthesiol. 2002, 19, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Işcan, G.; Kirimer, N.; Kürkcüoğlu, M.; Başer, K.H.; Demirci, F. Antimicrobial screening of Mentha piperita essential oils. J. Agric. Food Chem. 2002, 50, 3943–3946. [Google Scholar] [CrossRef]
- Rozza, A.L.; Hiruma-Lima, C.A.; Takahira, R.K.; Padovani, C.R.; Pellizzon, C.H. Effect of menthol in experimentally induced ulcers: Pathways of gastroprotection. Chem-Biol. Interact. 2013, 206, 272–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozza, A.L.; Meira de Faria, F.; Souza Brito, A.R.; Pellizzon, C.H. The gastroprotective effect of menthol: Involvement of anti-apoptotic, antioxidant and anti-inflammatory activities. PLoS ONE 2014, 9, e86686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Gramma, L.S.D.; Marques, F.M.; Vittorazzi, C.; de Andrade, T.A.; Frade, M.A.; de Andrade, T.U.; Endringer, D.C.; Scherer, R.; Fronza, M. Struthanthus vulgaris ointment prevents an over expression of inflammatory response and accelerates the cutaneous wound healing. J. Ethnopharmacol. 2016, 190, 319–327. [Google Scholar] [CrossRef]
- Arunachalam, K.; Parimelazhagan, T. Anti-inflammatory, wound healing and in-vivo antioxidant properties of the leaves of Ficus amplissima Smith. J. Ethnopharmacol. 2013, 145, 139–145. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hawkins, R.E.; Brian, M.; Carrell, R.W. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975, 85, 337–341. [Google Scholar]
- Yoshikawa, T.; Naito, Y.; Kishi, A.; Tomii, T.; Kaneko, T.; Iinuma, S.; Ichikawa, H.; Yasuda, M.; Takahashi, S.; Kondo, M. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut 1993, 34, 732–737. [Google Scholar] [CrossRef] [Green Version]
- Carlberg, I.; Mannervick, B. Glutathione reductase. Methods Enzym. 1985, 113, 484–499. [Google Scholar] [CrossRef]
- Faure, P.; Lafond, J.L. Measurement of plasma sulfhydryl and carbonyl groups as a possible indicator of protein oxidation. In Analysis of Free Radicals in Biological Systems, 1st ed.; Birkhäauser: Basel, Switzerland, 1995; pp. 237–248. [Google Scholar]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef]
- Nóbrega, R.H.; Greebe, C.D.; van de Kant, H.; Bogerd, J.; de Franca, L.R.; Schulz, R.W. Spermatogonial stem cell niche and spermatogonial stem cell transplantation in zebrafish. PLoS ONE 2010, 5, e12808. [Google Scholar] [CrossRef] [Green Version]
- Vischer, H.F.; Teves, A.C.; Ackermans, J.C.; van Dijk, W.; Schulz, R.W.; Bogerd, J. Cloning and spatiotemporal expression of the follicle-stimulating hormone beta subunit complementary DNA in the African catfish (Clarias gariepinus). Biol. Reprod. 2003, 68, 1324–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanathan, G.; Muthukumar, T.; Tirichurapalli Sivagnanam, U. In vivo efficiency of the collagen coated nanofibrous scaffold and their effect on growth factors and pro-inflammatory cytokines in wound healing. Eur. J. Pharm. 2017, 814, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.L.; Calpena, A.C.; Rodríguez-Lagunas, M.J.; Domènech, Ò.; Bozal-de Febrer, N.; Garduño-Ramírez, M.L.; Clares, B. Endogenous antioxidant cocktail loaded hydrogel for topical wound healing of burns. Pharmaceutics 2020, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Kozyreva, T.V.; Khramova, G.M.; Voronova, I.P.; Evtushenko, A.A. The influence of cooling and TRPM8 ion channel activation on the level of pro-inflammatory cytokines in normotensive and hypertensive rats. J. Biol. 2016, 61, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Modarresi, M.; Farahpour, M.R.; Baradaran, B. Topical application of Mentha piperita essential oil accelerates wound healing in infected mice model. Inflammopharmacology 2019, 27, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Babamohamadi, H.; Ansari, Z.; Nobahar, M.; Mirmohammadkhani, M. The effects of peppermint gel on prevention of pressure injury in hospitalized patients with head trauma in neurosurgical ICU: A double-blind randomized controlled trial. Complement. Med. 2019, 47, 102223. [Google Scholar] [CrossRef]
- Ud-Din, S.; Wilgus, T.A.; McGeorge, D.D.; Bayat, A. Pre-emptive priming of human skin improves cutaneous scarring and is superior to immediate and delayed topical anti-scarring treatment post-wounding: A double-blind randomised placebo-controlled clinical trial. Pharmaceutics 2021, 13, 510. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The role of oxidative stress and antioxidants in diabetic wound healing. Oxid. Med. Cell Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Abdel-Naim, A.B.; Alarif, W.M.; Algandaby, M.M.; Alburae, N.A.; Alghamdi, A.M.; Nasrullah, M.Z.; Fahmy, U.A. Euryops arabicus promotes healing of excised wounds in rat skin: Emphasis on its collagen-enhancing, antioxidant, and anti-inflammatory activities. Oxid. Med. Cell Longev. 2021, 2021, 8891445. [Google Scholar] [CrossRef]
- Murthy, S.; Gautam, M.K.; Goel, S.; Purohit, V.; Sharma, H.; Goel, R.K. Evaluation of in vivo wound healing activity of Bacopa monniera on different wound model in rats. Biomed. Res. Int. 2013, 2013, 972028. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [Green Version]
- Al-Dhuayan, I.; Kotb, E.; Alqosaibi, A.; Mahmoud, A. Histological studies on a newly isolated Bacillus subtilis D10 protease in the debridement of burn wound eschars using mouse model. Pharmaceutics 2021, 13, 923. [Google Scholar] [CrossRef]
- Dorjsembe, B.; Lee, H.J.; Kim, M.; Dulamjav, B.; Jigjid, T.; Nho, C.W. Achillea asiatica extract and its active compounds induce cutaneous wound healing. J. Ethnopharmacol. 2017, 206, 306–314. [Google Scholar] [CrossRef]
- Bastaki, S.M.; Adeghate, E.; Amir, N.; Ojha, S.; Oz, M. Menthol inhibits oxidative stress and inflammation in acetic acid-induced colitis in rat colonic mucosa. Am. J. Transl. Res. 2018, 10, 4210–4222. [Google Scholar] [PubMed]
- Wang, Q.; Yang, Y.; Chen, K.L.; Tang, B.; Peng, K.; Wang, Z.; Yang, P.; Yang, D.; Yang, Y. Dietary menthol attenuates inflammation and cardiac remodeling after myocardial infarction via the Transient Receptor Potential Melastatin 8. Am. J. Hypertens 2019, 33, 223–233. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in wound healing: A comprehensive review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, P.I.S.; Estevão, M.D.; Redruello, B.; Socorro, S.M.; Canário, A.V.M.; Power, D.M. Immunohistochemical detection of estrogen receptors in fish scales. Gen. Comp. Endocrinol. 2009, 160, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Kubo, H.; Hayashi, T.; Ago, K.; Ago, M.; Kanekura, T.; Ogata, M. Temporal expression of wound healing-related genes in skin burn injury. Leg Med. 2014, 16, 8–13. [Google Scholar] [CrossRef]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Anti-inflammatory effect of Garcinia mangostana Linn. pericarp extract in methicillin-resistant Staphylococcus aureus-induced superficial skin infection in mice. Biomed. Pharm. 2019, 111, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Li, X.; Ye, Y.; Shu, X.; Gong, J.; Wang, J. Castanea mollissima shell prevents an over expression of inflammatory response and accelerates the dermal wound healing. J. Ethnopharmacol. 2018, 220, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.; Singh, V.; Kumawat, S.; Kumar, D.; Lingaraju, M.C.; Uttam Singh, T.; Rahal, A.; Tandan, S.K.; Kumar, D. Deferoxamine modulates cytokines and growth factors to accelerate cutaneous wound healing in diabetic rats. Eur. J. Pharm. 2015, 764, 9–21. [Google Scholar] [CrossRef]
- Kaisang, L.; Siyu, W.; Lijun, F.; Daoyan, P.; Xian, C.J.; Jie, S. Adipose-derived stem cells seeded in Pluronic F-127 hydrogel promotes diabetic wound healing. J. Surg. Res. 2017, 217, 63–74. [Google Scholar] [CrossRef] [PubMed]




| Gene | Size (bp) | Sequence 5′–3′ | MT | NCBI Reference Sequence |
|---|---|---|---|---|
| Il-1β | 93 | FW: AGGCTTCCTTGTGCAAGTGT | 60 °C | NM_031512.2 |
| RV: AGGTCATTCTCCTCACTGTCG | ||||
| Il-6 | 92 | FW: TCATTCTGTCTCGAGCCCAC | 60 °C | NM_012589.2 |
| RV: CTCCGCAAGAGACTTCCAGC | ||||
| Il-10 | 95 | FW: GACGCTGTCATCGATTTCTCC | 60 °C | NM_012854.2 |
| RV: GCTCCAAGACAAAGGTGTCTAC | ||||
| Tnf-α | 100 | FW: ATGGGCTCCCTCTCATCAGT | 60 °C | NM_012675.3 |
| RV: TGGTTTGCTACGACGTGGG | ||||
| Ki-67 | 100 | FW: GGGTTTCCAGACACCAGACC | 60 °C | NM_001271366.1 |
| RV: CCAGGAAGACCAGTTAGAACC | ||||
| Ef-1α | 91 | FW: CTTTGGACTGCATTCTGCCG | 60 °C | NM_175838.1 |
| RV: GTGCCAATGCCGCCAATTTT |
| Period | Treatment | GSH | GR | GPx | SOD | MPO |
|---|---|---|---|---|---|---|
| 3 days | Vehicle | 3.29 ± 0.28 | 57.61 ± 2.89 | 26.50 ± 4.17 | 33.21 ± 2.07 | 0.23 ± 0.03 |
| Collagenase | 3.72 ± 0.46 | 60.12 ± 3.52 | 22.99 ± 3.20 | 31.17 ± 1.87 | 0.35 ± 0.05 | |
| ME0.5 | 2.46 ± 0.22 | 58.20 ± 0.88 | 24.27 ± 2.87 | 32.13 ± 3.74 | 0.39 ± 0.01 | |
| 7 days | Vehicle | 3.43 ± 0.35 | 31.93 ± 4.14 | 19.10 ± 3.03 | 36.42 ± 2.63 | 0.12 ± 0.01 |
| Collagenase | 6.01 ± 0.93 | 34.28 ± 3.66 | 21.76 ± 2.89 | 41.63 ± 4.32 | 0.10 ± 0.00 | |
| ME0.5 | 11.39 ± 0.92 *** | 48.83 ± 4.12 * | 31.73 ± 2.27 * | 50.86 ± 5.82 * | 0.11 ± 0.01 | |
| 14 days | Vehicle | 13.65 ± 2.18 | 96.34 ± 4.01 | 62.88 ± 3.57 | 31.31 ± 2.54 | 0.47 ± 0.09 |
| Collagenase | 12.03 ± 0.75 | 97.00 ± 4.55 | 58.20 ± 2.86 | 34.48 ± 1.46 | 0.30 ± 0.02 | |
| ME0.5 | 10.82 ± 1.00 | 82.89 ± 2.57 | 53.31 ± 1.80 | 27.35 ± 0.45 | 0.24 ± 0.02 * |
| Period | Treatment | IL-1β | IL-6 | TNF-α | IL-10 |
|---|---|---|---|---|---|
| 3 days | Vehicle | 1238.00 ± 137.90 | 2083.00 ± 219.00 | 205.50 ± 34.11 | 203.60 ± 66.43 |
| Collagenase | 1176.00 ± 186.60 | 1730.00 ± 264.30 | 202.70 ± 64.39 | 181.70 ± 51.34 | |
| ME0.5 | 1177.00 ± 226.50 | 1505.00 ± 308.10 | 120.10 ± 27.55 | 397.60 ± 158.90 | |
| 7 days | Vehicle | 807.20 ± 165.80 | 1893.00 ± 566.80 | 80.35 ± 27.41 | 144.90 ± 35.17 |
| Collagenase | 579.50 ± 104.80 | 820.80 ± 218.70 * | 45.33 ± 13.75 | 457.50 ± 149.70 | |
| ME0.5 | 266.60 ± 68.05 * | 570.10 ± 98.06 ** | 21.19 ± 4.03 * | 262.30 ± 78.13 | |
| 14 days | Vehicle | 2230.00 ± 254.70 | 1653.00 ± 177.30 | 138.30 ± 40.96 | 217.10 ± 70.60 |
| Collagenase | 1369.00 ± 207.70 * | 1113.00 ± 149.00 | 30.52 ± 8.73 ** | 645.50 ± 156.80 | |
| ME0.5 | 937.40 ± 142.20 *** | 831.70 ± 173.30 ** | 31.58 ± 10.02 ** | 1128.00 ± 161.80 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozza, A.L.; Beserra, F.P.; Vieira, A.J.; Oliveira de Souza, E.; Hussni, C.A.; Martinez, E.R.M.; Nóbrega, R.H.; Pellizzon, C.H. The Use of Menthol in Skin Wound Healing—Anti-Inflammatory Potential, Antioxidant Defense System Stimulation and Increased Epithelialization. Pharmaceutics 2021, 13, 1902. https://doi.org/10.3390/pharmaceutics13111902
Rozza AL, Beserra FP, Vieira AJ, Oliveira de Souza E, Hussni CA, Martinez ERM, Nóbrega RH, Pellizzon CH. The Use of Menthol in Skin Wound Healing—Anti-Inflammatory Potential, Antioxidant Defense System Stimulation and Increased Epithelialization. Pharmaceutics. 2021; 13(11):1902. https://doi.org/10.3390/pharmaceutics13111902
Chicago/Turabian StyleRozza, Ariane Leite, Fernando Pereira Beserra, Ana Júlia Vieira, Eduardo Oliveira de Souza, Carlos Alberto Hussni, Emanuel Ricardo Monteiro Martinez, Rafael Henrique Nóbrega, and Cláudia Helena Pellizzon. 2021. "The Use of Menthol in Skin Wound Healing—Anti-Inflammatory Potential, Antioxidant Defense System Stimulation and Increased Epithelialization" Pharmaceutics 13, no. 11: 1902. https://doi.org/10.3390/pharmaceutics13111902