Evaluation of Aminopolycarboxylate Chelators for Whole-Body Clearance of Free 225Ac: A Feasibility Study to Reduce Unexpected Radiation Exposure during Targeted Alpha Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Radionuclides
2.2. Preparation of Reagents
2.3. In Vitro Analysis of 225Ac Chelates Formation
2.4. Animals
2.5. Biodistribution of Free 225Ac in Mice and Dosimetry Analysis
2.6. In Vivo Chelating Study
2.6.1. Experiment 1
2.6.2. Experiment 2
2.7. Statistical Analysis
3. Results
3.1. In Vitro Analysis of 225Ac Chelates Formation
3.2. Biodistribution and Dosimetry of 225Ac in Mice
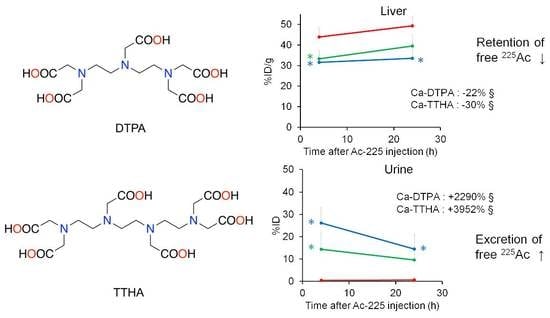
3.3. Effect of Chelator Administration In Vivo
3.3.1. Experiment 1
3.3.2. Experiment 2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miederer, M.; Scheinberg, D.A.; McDevitt, M.R. Realizing the Potential of the Actinium-225 Radionuclide Generator in Targeted Alpha Particle Therapy Applications. Adv. Drug Deliv. Rev. 2008, 60, 1371–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheinberg, D.A.; McDevitt, M.R. Actinium-225 in Targeted Alpha-Particle Therapeutic Applications. Curr. Radiopharm. 2011, 4, 306–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted Alpha-Therapy of Metastatic Castration-Resistant Prostate Cancer With 225Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juneau, D.; Saad, F.; Berlin, A.; Metser, U.; Puzanov, I.; Lamonica, D.; Sparks, R.; Burak, E.; Simms, R.; Rhoden, J.; et al. Preliminary Dosimetry Results From a First-In-Human Phase I Study Evaluating the Efficacy and Safety of [225Ac]-FPI-1434 in Patients With IGF-1R Expressing Solid Tumors. J. Nucl. Med. 2021, 62, 74. [Google Scholar]
- Garg, R.; Allen, K.J.H.; Dawicki, W.; Geoghegan, E.M.; Ludwig, D.L.; Dadachova, E. 225Ac-Labeled CD33-Targeting Antibody Reverses Resistance to Bcl-2 Inhibitor Venetoclax in Acute Myeloid Leukemia Models. Cancer Med. 2021, 10, 1128–1140. [Google Scholar] [CrossRef]
- Eychenne, R.; Chérel, M.; Haddad, F.; Guérard, F.; Gestin, J.F. Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The “Hopeful Eight”. Pharmaceutics 2021, 13, 906. [Google Scholar] [CrossRef]
- Deal, K.A.; Davis, I.A.; Mirzadeh, S.; Kennel, S.J.; Brechbiel, M.W. Improved In Vivo Stability of Actinium-225 Macrocyclic Complexes. J. Med. Chem. 1999, 42, 2988–2992. [Google Scholar] [CrossRef]
- Thiele, N.A.; Wilson, J.J. Actinium-225 for Targeted Alpha Therapy: Coordination Chemistry and Current Chelation Approaches. Cancer Biother. Radiopharm. 2018, 33, 336–348. [Google Scholar] [CrossRef]
- Davis, I.A.; Glowienka, K.A.; Boll, R.A.; Deal, K.A.; Brechbiel, M.W.; Stabin, M.; Bochsler, P.N.; Mirzadeh, S.; Kennel, S.J. Comparison of 225Actinium Chelates: Tissue Distribution and Radiotoxicity. Nucl. Med. Biol. 1999, 26, 581–589. [Google Scholar] [CrossRef]
- Jiang, Z.; Revskaya, E.; Fisher, D.R.; Dadachova, E. In Vivo Evaluation of Free and Chelated Accelerator-Produced Actinium-225—Radiation Dosimetry and Toxicity Results. Curr. Radiopharm. 2018, 11, 215–222. [Google Scholar] [CrossRef]
- Miederer, M.; Henriksen, G.; Alke, A.; Mossbrugger, I.; Quintanilla-Martinez, L.; Senekowitsch-Schmidtke, R.; Essler, M. Preclinical Evaluation of the Alpha-Particle Generator Nuclide 225Ac for Somatostatin Receptor Radiotherapy of Neuroendocrine Tumors. Clin. Cancer Res. 2008, 14, 3555–3561. [Google Scholar] [CrossRef] [Green Version]
- Kennel, S.J.; Chappell, L.L.; Dadachova, K.; Brechbiel, M.W.; Lankford, T.K.; Davis, I.A.; Stabin, M.; Mirzadeh, S. Evaluation of 225Ac for Vascular Targeted Radioimmunotherapy of Lung Tumors. Cancer Biother. Radiopharm. 2000, 15, 235–244. [Google Scholar] [CrossRef]
- Liu, Y.; Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Naka, S.; Ooe, K.; Toyoshima, A.; Nagata, K.; Haberkorn, U.; Kratochwil, C.; et al. Fibroblast Activation Protein Targeted Therapy Using [177Lu]FAPI-46 Compared With [225Ac]FAPI-46 in a Pancreatic Cancer Model. Eur. J. Nucl. Med. Mol. Imaging 2021, in press. [Google Scholar] [CrossRef]
- Austin, A.L.; Ellender, M.; Haines, J.W.; Harrison, J.D.; Lord, B.I. Microdistribution and Localized Dosimetry of the Alpha-Emitting Radionuclides 239Pu, 241Am and 233U in Mouse Femoral Shaft. Int. J. Radiat. Biol. 2000, 76, 101–111. [Google Scholar] [CrossRef]
- Hobbs, R.F.; Song, H.; Watchman, C.J.; Bolch, W.E.; Aksnes, A.K.; Ramdahl, T.; Flux, G.D.; Sgouros, G. A Bone Marrow Toxicity Model for 223Ra Alpha-Emitter Radiopharmaceutical Therapy. Phys. Med. Biol. 2012, 57, 3207–3222. [Google Scholar] [CrossRef] [Green Version]
- Walshe, J.M. Endogenous Copper Clearance in Wilson’s Disease: A Study of the Mode of Action of Penicillamine. Clin. Sci. 1964, 26, 461–469. [Google Scholar]
- Akorn. BAL in Oil Ampules (Dimercaprol Injection, USP); Label Information; Akorn: Lake Forest, IL, USA, 2017. [Google Scholar]
- Medics. Calcium Disodium Versenate (Edetate Calcium Disodium Injection, USP); Label Information; Graceway Pharmaceuticals: Bristol, TN, USA, 2009. [Google Scholar]
- Hameln Pharmaceuticals. Pentetate Calcium Trisodium Injection; Prescribing Information; Hameln Pharmaceuticals: Gloucester, UK, 2013. [Google Scholar]
- Ferrier, M.G.; Stein, B.W.; Batista, E.R.; Berg, J.M.; Birnbaum, E.R.; Engle, J.W.; John, K.D.; Kozimor, S.A.; Lezama Pacheco, J.S.; Redman, L.N. Synthesis and Characterization of the Actinium Aquo Ion. ACS Cent. Sci. 2017, 3, 176–185. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E. Critical Stability Constants, Enthalpies and Entropies for the Formation of Metal Complexes of Aminopolycarboxylic Acids and Carboxylic Acids. Sci. Total Environ. 1987, 64, 125–147. [Google Scholar] [CrossRef]
- Gauthier, J.P.; Deblonde, M.Z.; Kersting, A.B. The Coordination Properties and Ionic Radius of Actinium: A 120-Year-Old Enigma. Coord. Chem. Rev. 2021, 446, 214130. [Google Scholar] [CrossRef]
- Clough, T.J.; Jiang, L.; Wong, K.L.; Long, N.J. Ligand Design Strategies to Increase Stability of Gadolinium-Based Magnetic Resonance Imaging Contrast Agents. Nat. Commun. 2019, 10, 1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An Overview of Targeted Alpha Therapy With 225Actinium and 213Bismuth. Curr. Radiopharm. 2018, 11, 200–208. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, M.R.; Ma, D.; Simon, J.; Frank, R.K.; Scheinberg, D.A. Design and Synthesis of 225Ac Radioimmunopharmaceuticals. Appl. Radiat. Isot. 2002, 57, 841–847. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching Chelators to Radiometals for Radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef]
- McAlister, D.R.; Horwitz, E.P. Chromatographic Generator Systems for the Actinides and Natural Decay Series Elements. Radiochim. Acta 2011, 99, 151–159. [Google Scholar] [CrossRef]
- Kannengießer, S. Optimization of the Synthesis of Ac-225-Labelled DOTA-Radioimmunoconjugates for Targeted Alpha Therapy, Based on Investigations on the Complexation of Trivalent Actinides by DOTA. Ph.D. Thesis, Ruprecht-Karls-Universität, Heidelberg, Germany, 2013. [Google Scholar]
- Kirschner, A.S.; Ice, R.D.; Beierwaltes, W.H. Author’s Reply: Radiation Dosimetry of 131-I-19-Iodocholesterol: The Pitfalls of Using Tissue Concentration Data. J. Nucl. Med. 1975, 16, 248–249. [Google Scholar]
- Stabin, M.G.; Sparks, R.B.; Crowe, E. OLINDA/EXM: The Second-Generation Personal Computer Software for Internal Dose Assessment in Nuclear Medicine. J. Nucl. Med. 2005, 46, 1023–1027. [Google Scholar]
- Barron, E.S.; Miller, Z.B.; Meyer, J. The Effect of 2:3-dimercaptopropanol on the Activity of Enzymes and on the Metabolism of Tissues. Biochem. J. 1947, 41, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshii, Y.; Matsumoto, H.; Yoshimoto, M.; Furukawa, T.; Morokoshi, Y.; Sogawa, C.; Zhang, M.R.; Wakizaka, H.; Yoshii, H.; Fujibayashi, Y.; et al. Controlled Administration of Penicillamine Reduces Radiation Exposure in Critical Organs During 64Cu-ATSM Internal Radiotherapy: A Novel Strategy for Liver Protection. PLoS ONE 2014, 9, e86996. [Google Scholar] [CrossRef]
- Morgan, R.M.; Smith, H. Histological Changes in Kidney, Liver and Duodenum of the Mouse Following the Acute and Subacute Administration of Diethylenetriaminepentaacetic Acid. Toxicology 1974, 2, 153–163. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Choppin, G.R. Structure and Thermodynamics of Lanthanide and Actinide Complexes in Solution. Pure Appl. Chem. 1971, 27, 23–42. [Google Scholar] [CrossRef] [Green Version]
- Martell, A.E.; Smith, R.M. Critical Stability Constants; Volume 1 (Amino Acids); Plenum Press: New York, NY, USA, 1974. [Google Scholar]
- Nash, K.L.; Brigham, D.; Shehee, T.C.; Martin, A. The Kinetics of Lanthanide Complexation by EDTA and DTPA in Lactate Media. Dalton Trans. 2012, 41, 14547–14556. [Google Scholar] [CrossRef] [PubMed]
- Kálmán, F.K.; Tircsó, G. Kinetic Inertness of the Mn2+ Complexes Formed With AAZTA and Some Open-Chain EDTA Derivatives. Inorg. Chem. 2012, 51, 10065–10067. [Google Scholar] [CrossRef] [Green Version]
- Szilágyi, E.B.; Brücher, E. Kinetics and Mechanisms of Formation of the Lanthanide (III) trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetate Comlexes. J. Chem. Soc. Dalton Trans. 2000, 13, 2229–2233. [Google Scholar] [CrossRef]
- Aaseth, J.; Crisponi, G.; Andersen, O. Chelation Therapy in the Treatment of Metal Intoxication; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Tollefson, A.D.; Smith, C.M.; Carpenter, M.H.; Croce, M.P.; Fassbender, M.E.; Koehler, K.E.; Lilley, L.M.; O’Brien, E.M.; Schmidt, D.R.; Stein, B.W.; et al. Measurement of 227Ac Impurity in 225Ac Using Decay Energy Spectroscopy. Appl. Radiat. Isot. 2021, 172, 109693. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted Alpha-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [Green Version]
- McDevitt, M.R.; Ma, D.; Lai, L.T.; Simon, J.; Borchardt, P.; Frank, R.K.; Wu, K.; Pellegrini, V.; Curcio, M.J.; Miederer, M.; et al. Tumor Therapy With Targeted Atomic Nanogenerators. Science 2001, 294, 1537–1540. [Google Scholar] [CrossRef]
- Miyamoto, T.; DeRose, R.; Suarez, A.; Ueno, T.; Chen, M.; Sun, T.P.; Wolfgang, M.J.; Mukherjee, C.; Meyers, D.J.; Inoue, T. Rapid and Orthogonal Logic Gating With a Gibberellin-Induced Dimerization System. Nat. Chem. Biol. 2012, 8, 465–470. [Google Scholar] [CrossRef]






| Chelators | Abbreviations | Providers | Dose | Administration Volume and Route |
|---|---|---|---|---|
| D-Penicillamine | - | Fujifilm Wako Chemicals | 300 mg/kg | 150 µL p.o. |
| 2,3-Dimercapto-1-propanol | Dimercaprol | Tokyo Chemical Industries | 60 mg/kg | 50 µL i.m. |
| Calcium diethylenetriamine-N,N,N′,N″,N″-pentaacetate | Ca-DTPA | Chemisch-pharmazeutische Fabrik GmbH | 150 mg/kg | 100 µL i.p. |
| Calcium ethylenediamine-N,N,N′,N′-tetraacetate | Ca-EDTA | Fujifilm Wako Chemicals | 150 mg/kg | 100 µL i.p. |
| trans-1,2-Diaminocyclohexane-N,N,N′,N′-tetraacetic acid | CyDTA | Fujifilm Wako Chemicals | 150 mg/kg | 100 µL i.p. |
| O,O’-Bis(2-aminoethyl)ethyleneglycol-N,N,N′,N′-tetraacetic acid | GEDTA | Fujifilm Wako Chemicals | 150 mg/kg | 100 µL i.p. |
| Triethylenetetramine-N,N,N′,N″,N‴,N‴-hexaacetic acid | TTHA | Fujifilm Wako Chemicals | 150 mg/kg | 100 µL i.p. |
| Calcium triethylenetetramine-N,N,N′,N″,N‴,N‴-hexaacetate | Ca-TTHA | See text | 150 mg/kg | 100 µL i.p. |
| 1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic acid | DO3A | Macrocyclics | 150 mg/kg | 100 µL i.p. |
| Estimated Absorbed Dose (mSvRBE5/MBq) | ||
|---|---|---|
| Target Organ | Male | Female |
| Adrenals | 2.34 × 102 | 3.03 × 102 |
| Brain | 5.62 | 6.66 |
| Breasts | 2.34 × 102 | 3.03 × 102 |
| Gallbladder wall | 2.34 × 102 | 3.03 × 102 |
| Lower large intestinal wall | 2.34 × 102 | 3.03 × 102 |
| Small intestine | 2.34 × 102 | 3.03 × 102 |
| Stomach wall | 2.34 × 102 | 3.03 × 102 |
| Upper large intestinal wall | 2.34 × 102 | 3.03 × 102 |
| Heart wall | 3.85 × 102 | 5.06 × 102 |
| Kidneys | 2.15 × 102 | 2.33 × 102 |
| Liver | 4.76 × 103 | 6.50 × 103 |
| Lungs | 1.61 × 102 | 2.01 × 102 |
| Muscle | 2.86 × 101 | 4.70 × 101 |
| Ovaries | 2.34 × 102 | 3.03 × 102 |
| Pancreas | 4.79 × 101 | 5.33 × 101 |
| Red marrow | 3.37 × 102 | 3.89 × 102 |
| Osteogenic cells | 1.17 × 104 | 1.63 × 104 |
| Skin | 2.34 × 102 | 3.03 × 102 |
| Spleen | 3.79 × 102 | 4.62 × 102 |
| Testes | 2.34 × 102 | |
| Thymus | 2.34 × 102 | 3.03 × 102 |
| Thyroid | 2.34 × 102 | 3.03 × 102 |
| Urinary bladder wall | 2.36 × 102 | 3.05 × 102 |
| Uterus | 2.34 × 102 | 3.03 × 102 |
| Total body | 4.45 × 102 | 5.78 × 102 |
| Effective dose equivalent | 8.72 × 102 | 1.17 × 103 |
| Effective dose | 5.64 × 102 | 7.52 × 102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimoto, M.; Yoshii, Y.; Matsumoto, H.; Shinada, M.; Takahashi, M.; Igarashi, C.; Hihara, F.; Tachibana, T.; Doi, A.; Higashi, T.; et al. Evaluation of Aminopolycarboxylate Chelators for Whole-Body Clearance of Free 225Ac: A Feasibility Study to Reduce Unexpected Radiation Exposure during Targeted Alpha Therapy. Pharmaceutics 2021, 13, 1706. https://doi.org/10.3390/pharmaceutics13101706
Yoshimoto M, Yoshii Y, Matsumoto H, Shinada M, Takahashi M, Igarashi C, Hihara F, Tachibana T, Doi A, Higashi T, et al. Evaluation of Aminopolycarboxylate Chelators for Whole-Body Clearance of Free 225Ac: A Feasibility Study to Reduce Unexpected Radiation Exposure during Targeted Alpha Therapy. Pharmaceutics. 2021; 13(10):1706. https://doi.org/10.3390/pharmaceutics13101706
Chicago/Turabian StyleYoshimoto, Mitsuyoshi, Yukie Yoshii, Hiroki Matsumoto, Mitsuhiro Shinada, Masashi Takahashi, Chika Igarashi, Fukiko Hihara, Tomoko Tachibana, Ayano Doi, Tatsuya Higashi, and et al. 2021. "Evaluation of Aminopolycarboxylate Chelators for Whole-Body Clearance of Free 225Ac: A Feasibility Study to Reduce Unexpected Radiation Exposure during Targeted Alpha Therapy" Pharmaceutics 13, no. 10: 1706. https://doi.org/10.3390/pharmaceutics13101706