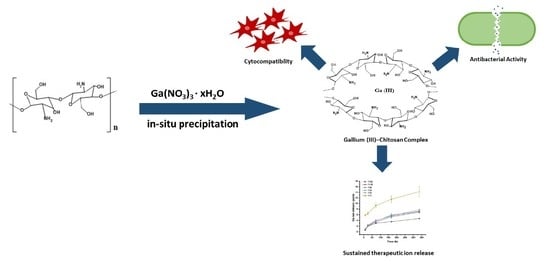
Facile Synthesis of Gallium (III)-Chitosan Complexes as Antibacterial Biomaterial
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation Gallium (III)-Chitosan Complex
| Ga (III): NH2 | CS | 1:32 | 1:16 | 1:8 | 1:4 | 1:2 | 1:1 |
|---|---|---|---|---|---|---|---|
| X [%] | 0 | 3 | 6 | 12 | 24 | 48 | 96 |
| 0 | 21 | 42 | 84 | 164 | 328 | 656 |
2.3. Characterizations
2.3.1. Morphological and Compositional Characterization
2.3.2. X-ray Diffraction
2.3.3. Release Studies
2.3.4. Wettability
2.3.5. Antibacterial Assay
2.3.6. Cell Studies
2.4. Statistical Analysis
3. Results and Discussion
3.1. Morphological and Compositional Characterization
3.2. Confirmation of Complexation by XRD
3.3. Ion Release from Complexes
3.4. Wettability
3.5. Antibacterial Activity
3.6. Cell Culture Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gimeno, M.; Pinczowski, P.; Pérez, M.; Giorello, A.; Martínez, M.Á.; Santamaría, J.; Arruebo, M.; Luján, L. A controlled antibiotic release system to prevent orthopedic-implant associated infections: An in vitro study. Eur. J. Pharm. Biopharm. 2015, 96, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Shuman, H.A.; Becker, L.; Aitken, M.L.; Caldwell, E.; Wilhelm, E.; Siehnel, R.J.; Singh, P.K.; Britigan, B.E.; Ravishankar, S.; et al. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10, 460. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Goy, R.C.; Britto, D.D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polímeros Ciência Tecnol. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Pestov, A.; Bratskaya, S. Chitosan and its derivatives as highly efficient polymer ligands. Molecules 2016, 21, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Chauhan, S. Modification of chitosan for sorption of metal ions. J. Chem. Pharm. Res. 2015, 7, 49–55. [Google Scholar]
- Gerente, C.; Lee, V.K.C.; Le Cloirec, P.; McKay, G. Application of chitosan for the removal of metals from wastewaters by adsorption—Mechanisms and models review. Crit. Rev. Environ. Sci. Technol. 2007, 37, 41–127. [Google Scholar] [CrossRef]
- Rashid, S.; Shen, C.; Chen, X.; Li, S.; Chen, Y.; Wen, Y.; Liu, J. Enhanced catalytic ability of chitosan-Cu-Fe bimetal complex for the removal of dyes in aqueous solution. RSC Adv. 2015, 5, 90731–90741. [Google Scholar] [CrossRef]
- Gritsch, L.; Lovell, C.; Goldmann, W.H.; Boccaccini, A.R. Fabrication and characterization of copper(II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr. Polym. 2018, 179, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Ramesan, V.S.; Jain, S. Chitosan-Glycerol Gel as Barrier Formulation for Metal Allergy. ACS Omega 2019, 4, 5900–5903. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, Y.; Fan, L.; Liu, H.; Hu, Y. Chitosan-metal complexes as antimicrobial agent: Synthesis, characterization and Structure-activity study. Polym. Bull. 2005, 55, 105–113. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, T.; Zhao, C. Preparation of chitosan-nylon-6 blended membranes containing silver ions as antibacterial materials. Carbohydr. Res. 2008, 343, 230–237. [Google Scholar] [CrossRef]
- Higazy, A.; Hashem, M.; ElShafei, A.; Shaker, N.; Hady, M.A. Development of antimicrobial jute packaging using chitosan and chitosan–metal complex. Carbohydr. Polym. 2010, 79, 867–874. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Chitambar, C.R. Medical applications and toxicities of gallium compounds. Int. J. Environ. Res. Public Health 2010, 7, 2337–2361. [Google Scholar] [CrossRef] [Green Version]
- Bonchi, C.; Imperi, F.; Minandri, F.; Visca, P.; Frangipani, E. Repurposing of gallium-based drugs for antibacterial therapy. BioFactors 2014, 40, 303–312. [Google Scholar] [CrossRef]
- Cai, X.; Cai, J.; Ma, K.; Huang, P.; Gong, L.; Huang, D.; Jiang, T.; Wang, Y. Fabrication and characterization of Mg-doped chitosan–gelatin nanocompound coatings for titanium surface functionalization. J. Biomater. Sci. Polym. Ed. 2016, 27, 954–971. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, X.; Chen, X.; Chen, Z.; Xia, Z. Antimicrobial effect of gallium nitrate against bacteria encountered in burn wound infections. RSC Adv. 2017, 7, 52266–52273. [Google Scholar] [CrossRef] [Green Version]
- Hijazi, S.; Visaggio, D.; Pirolo, M.; Frangipani, E.; Bernstein, L.; Visca, P. Antimicrobial activity of gallium compounds on ESKAPE pathogens. Front. Cell. Infect. Microbiol. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kelson, A.B.; Carnevali, M.; Truong-Le, V. Gallium-based anti-infectives: Targeting microbial iron-uptake mechanisms. Curr. Opin. Pharmacol. 2013, 13, 707–716. [Google Scholar] [CrossRef]
- Shen, C.; Chen, H.; Wu, S.; Wen, Y.; Li, L.; Jiang, Z.; Li, M.; Liu, W. Highly efficient detoxification of Cr(VI) by chitosan-Fe(III) complex: Process and mechanism studies. J. Hazard. Mater. 2013, 244–245, 689–697. [Google Scholar] [CrossRef]
- Qu, J.; Hu, Q.; Shen, K.; Zhang, K.; Li, Y.; Li, H.; Zhang, Q.; Wang, J.; Quan, W. The preparation and characterization of chitosan rods modified with Fe 3+ by a chelation mechanism. Carbohydr. Res. 2011, 346, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Akhlaghi, M.; Jalilian, A.R. Preparation and evaluation of a [66ga] gallium chitosan complex in fibrosarcoma bearing animal models. Nukleonika 2011, 56, 35–40. [Google Scholar]
- Akhtar, M.A.; Hadzhieva, Z.; Dlouhý, I.; Boccaccini, A.R. Electrophoretic deposition and characterization of functional coatings based on an antibacterial gallium (III)-chitosan complex. Coatings 2020, 10, 483. [Google Scholar] [CrossRef]
- Trimukhe, K.D.; Varma, A.J. A morphological study of heavy metal complexes of chitosan and crosslinked chitosans by SEM and WAXRD. Carbohydr. Polym. 2008, 71, 698–702. [Google Scholar] [CrossRef]
- Timur, M.; Paş, A.; Paşa, A. Synthesis, Characterization, Swelling, and Metal Uptake Studies of Aryl Cross-Linked Chitosan Hydrogels. ACS Omega 2018, 3, 17416–17424. [Google Scholar] [CrossRef]
- Rogina, A.; Lončarević, A.; Antunović, M.; Marijanović, I.; Ivanković, M.; Ivanković, H. Tuning physicochemical and biological properties of chitosan through complexation with transition metal ions. Int. J. Biol. Macromol. 2019, 129, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, X.; Xu, R.; Wang, W.; Liu, X.; Yeung, K.W.K.; Chu, P.K. Electrochemically deposited chitosan/Ag complex coatings on biomedical NiTi alloy for antibacterial application. Surf. Coat. Technol. 2013, 232, 370–375. [Google Scholar] [CrossRef]
- Antonino, R.S.C.M.D.Q.; Fook, B.R.P.L.; Lima, V.A.D.O.; Rached, R.Í.D.F.; Lima, E.P.N.; Lima, R.J.D.S.; Covas, C.A.P.; Fook, M.V.L. Preparation and characterization of chitosan obtained from shells of shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zhang, S.; Xia, L.; Ding, C.; Wen, L.; Wan, W. Biocompatible nanocarriers that respond to oxidative environments via interactions between chitosan and multiple metal ions. Int. J. Nanomed. 2016, 11, 2769–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Shen, Y.; Shen, C.; Wen, Y.; Liu, W. Al-doping chitosan-Fe(III) hydrogel for the removal of fluoride from aqueous solutions. Chem. Eng. J. 2014, 248, 98–106. [Google Scholar] [CrossRef]
- Modrzejewska, Z.; Dorabialska, M. The mechanism of sorption of Ag+ ions on chitosan microgranules: IR and NMR studies. Prog. Chem. Appl. Chitin Deriv. 2009, 14, 49–64. [Google Scholar]
- Brunel, F.; El Gueddari, N.E.; Moerschbacher, B.M. Complexation of copper(II) with chitosan nanogels: Toward control of microbial growth. Carbohydr. Polym. 2013, 92, 1348–1356. [Google Scholar] [CrossRef]
- Hussein, M.H.M.; El-Hady, M.F.; Sayed, W.M.; Hefni, H. Preparation of some chitosan heavy metal complexes and study of its properties. Polym. Sci. Ser. A 2012, 54, 113–124. [Google Scholar] [CrossRef]
- Rhazi, M.; Tolaimate, A.; Rhazi, M.; Desbriéres, J.; Tolaimate, A.; Rinaudo, M.; Alagui, A.; Vottero, P. Contribution to the study of the complexation of copper by chitosan and oligomers. Polymer 2002, 43, 1267–1276. [Google Scholar] [CrossRef]
- Gritsch, L.; Maqbool, M.; Mourin, V.; Ciraldo, F.E.; Cresswell, M.; Jackson, P.R.; Lovell, C.; Boccaccini, A.R. Chitosan/hydroxyapatite composite bone tissue engineering scaffolds with dual and decoupled therapeutic ion delivery: Copper and strontium. J. Mater. Chem. B 2019, 7, 6109–6124. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.A.; Ilyas, K.; Dlouhý, I.; Siska, F.; Boccaccini, A.R. Electrophoretic deposition of copper(II)-chitosan complexes for antibacterial coatings. Int. J. Mol. Sci. 2020, 21, 2637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Periayah, M.; Halim, A.; Saad, A.M. Chitosan: A promising marine polysaccharide for biomedical research. Pharmacogn. Rev. 2016, 10, 39. [Google Scholar] [CrossRef]
- Rhazi, M.; Desbrières, J.; Tolaimate, A.; Rinaudo, M.; Vottero, P.; Alagui, A.; El Meray, M. Influence of the nature of the metal ions on the complexation with chitosan. Application to the treatment of liquid waste. Eur. Polym. J. 2002, 38, 1523–1530. [Google Scholar] [CrossRef]
- Hernández, R.B.; Franco, A.P.; Yola, O.R.; López-Delgado, A.; Felcman, J.; Recio, M.A.L.; Mercê, A.L.R. Coordination study of chitosan and Fe3+. J. Mol. Struct. 2008, 877, 89–99. [Google Scholar] [CrossRef]
- Juang, R.S.; Shao, H.J. Effect of pH on competitive adsorption of Cu(II), Ni(II), and Zn(II) from water onto chitosan beads. Adsorption 2002, 8, 71–78. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; De La Caba, K. Functional properties of chitosan-based films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef]
- Tang, L.; Thevenot, P.; Hu, W. Surface Chemistry Influences Implant Biocompatibility. Curr. Top. Med. Chem. 2008, 8, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Valappil, S.P.; Ready, D.; Abou Neel, E.A.; Pickup, D.M.; Chrzanowski, W.; O’Dell, L.A.; Newport, R.J.; Smith, M.E.; Wilson, M.; Knowles, J.C. Antimicrobial gallium-doped phosphate-based glasses. Adv. Funct. Mater. 2008, 18, 732–741. [Google Scholar] [CrossRef]
- Minandri, F.; Bonchi, C.; Frangipani, E.; Imperi, F.; Visca, P. Promises and failures of gallium as an antibacterial agent. Future Microbiol. 2014, 9, 379–397. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnology 2017, 15, 1–20. [Google Scholar] [CrossRef]
- Javadzadeh, Y.; Hamedeyaz, S. Floating Drug Delivery Systems for Eradication of Helicobacter Pylori in Treatment of Peptic Ulcer Disease. In Trends in Helicobacter Pylori Infection; InTechOpen Ltd.: London, UK, 2014. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Chitambar, C.R. Role of Oxidative Stress in the Induction of Metallothionein-2A and Heme Oxygenase-1 Gene Expression by the Antineoplastic Agent Gallium Nitrate in Human Lymphoma Cells. Free. Radic. Biol. Med. 2008, 45, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Mohebbi, S.; Nezhad, M.N.; Zarrintaj, P.; Jafari, S.H.; Gholizadeh, S.S.; Saeb, M.R.; Mozafari, M. Chitosan in Biomedical Engineering: A Critical Review. Curr. Stem Cell Res. Ther. 2018, 14, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Milheiro, A.; Nozaki, K.; Kleverlaan, C.J.; Muris, J.; Miura, H.; Feilzer, A.J. In vitro cytotoxicity of metallic ions released from dental alloys. Odontology 2016, 104, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Best, M.G.; Cunha-Reis, C.; Ganin, A.Y.; Sousa, A.; Johnston, J.; Oliveira, A.L.; Smith, D.G.E.; Yiu, H.H.P.; Cooper, I.R. Antimicrobial Properties of Gallium(III)- and Iron(III)-Loaded Polysaccharides Affecting the Growth of Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa, In Vitro. ACS Appl. Bio Mater. 2020, 3, 7589–7597. [Google Scholar] [CrossRef]








| Ga (III): NH2 | 1:32 | 1:16 | 1:8 | 1:4 | 1:2 | 1:1 |
|---|---|---|---|---|---|---|
| GaLα/Ckα ratio [%] | 3.1 ± 0.2 | 7.1 ± 0.3 | 10.5 ± 0.4 | 32 ± 1 | 37 ± 2 | 93 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, M.A.; Hadzhieva, Z.; Ilyas, K.; Ali, M.S.; Peukert, W.; Boccaccini, A.R. Facile Synthesis of Gallium (III)-Chitosan Complexes as Antibacterial Biomaterial. Pharmaceutics 2021, 13, 1702. https://doi.org/10.3390/pharmaceutics13101702
Akhtar MA, Hadzhieva Z, Ilyas K, Ali MS, Peukert W, Boccaccini AR. Facile Synthesis of Gallium (III)-Chitosan Complexes as Antibacterial Biomaterial. Pharmaceutics. 2021; 13(10):1702. https://doi.org/10.3390/pharmaceutics13101702
Chicago/Turabian StyleAkhtar, Muhammad Asim, Zoya Hadzhieva, Kanwal Ilyas, Muhammad Saad Ali, Wolfgang Peukert, and Aldo R. Boccaccini. 2021. "Facile Synthesis of Gallium (III)-Chitosan Complexes as Antibacterial Biomaterial" Pharmaceutics 13, no. 10: 1702. https://doi.org/10.3390/pharmaceutics13101702