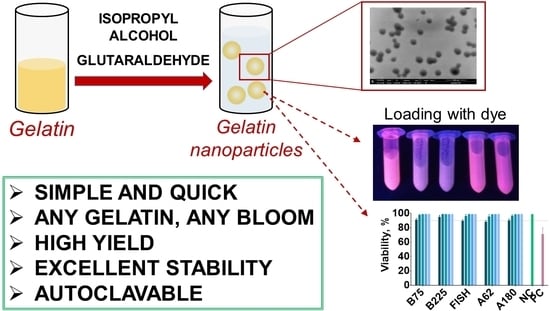
Modified Desolvation Method Enables Simple One-Step Synthesis of Gelatin Nanoparticles from Different Gelatin Types with Any Bloom Values
Abstract
:1. Introduction
- To confirm the effect of stirring on desolvation of gelatin;
- To study the influence of gelatin pH, concentration, and non-solvent type on the size and yield of gelatin nanoparticles;
- Using optimized conditions to synthesize nanoparticles from porcine, bovine, and fish gelatin with different bloom values (including lowest values available) in a hundreds-of-microgram scale;
- To study storage stability and colloidal stability of resulting nanoparticles;
- To load model hydrophobic molecules into gelatin nanoparticles;
- To assess the effect of sterilization on the integrity of gelatin nanoparticles;
- To study cytotoxicity of gelatin nanoparticles prepared by modified desolvation method.
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gelatin Stock Solutions
2.3. Synthesis of Gelatin Nanoparticles in Hundred-of-Milligram Scale
2.4. Preparation of Gelatin Nanoparticles Loaded with Fluorescent Europium Chelates
2.5. Steam Autoclaving
2.6. Assessment of Nanoparticle Stability at Different pH and High Salt Concentrations
2.7. Cell Viability Study
3. Results and Discussion
3.1. Stirring Promotes Nanoparticle Aggregation in the Course of Desolvation
3.2. Influence of pH, Gelatin Concentration, Type, and Volume of Desolvating Agent on the Size and Yield of Gelatin Nanoparticles
3.3. Synthesis of Nanoparticles from Different Gelatins in Hundreds-of-Milligram-Scale
3.3.1. Size, Zeta Potential, and Shape of Nanoparticles
3.3.2. Yield
3.3.3. Absorbance and Fluorescence Spectra of Gelatin Nanoparticles
3.4. Stability of Nanoparticles at Various pH and Salt Concentrations
3.5. Storage Stability
3.6. Loading of Gelatin Nanoparticles with Fluorescent Complex
3.7. Sterilization of Gelatin Nanoparticles
3.8. Effect of Gelatin Nanoparticles on the Viability of Peripheral Blood Mononuclear Cells
3.9. Future Perspectives and Limitations of This Study
- The size of all synthesized gelatin nanoparticles exceeded 100 nm. We did not obtain smaller nanoparticles, however, the desolvation method allows the preparation of nanoparticles whose diameter is less than 100 nm [23,26]. We suppose that a decrease of initial gelatin concentration or increase of gelatin solution pH is a possible way to obtain nanoparticles smaller than 100 nm.
- We did not prepare nanoparticles from gelatin solutions with concentrations higher than 20 mg/mL at a high scale. As we mentioned in Section 3.3, our goal was to prepare relatively small nanoparticles, less than 200 nm, which is possible by using smaller gelatin concentrations for all tested gelatin types. Data obtained in the course of optimization experiments and our previous results [30] both demonstrate that gelatin nanoparticles can be prepared at high starting gelatin concentrations. Variation of pH and volume of the desolvating agent is a possible way to decrease the size of nanoparticles when the concentration of gelatin is high.
- As we mentioned before, we did not remove endotoxin from gelatin nanoparticles nor examine endotoxin concentration in nanoparticle preparations prior to cell viability testing. Synthesis of apyrogenic nanoparticles is a challenging task, which requires a separate set of experiments and was, therefore, beyond the scope of the present work. We just note that the protocol of gelatin depyrogenation was previously reported by Singh et al. [37], besides, post-synthesis depyrogenation by gamma-irradiation also remains a possible option [72].
- The effect of several factors on the desolvation process was not studied: temperature of starting materials [49], salt concentration, acidic pH values, gelatin pre-incubation [84], the longevity of incubation with alcohols, and so on. Nevertheless, we performed preliminary experiments adding NaCl before desolvation. The addition of salt resulted in the formation of microparticles visible by the eye, however, a systematic study has not been conducted.
- Despite various types of animal gelatin being tested, we did not prepare nanoparticles from human recombinant gelatin. Application of natural gelatin from animal sources can be limited due to pathogen (first of all, prions) contamination, religious reasons, and its potential immunogenicity [4]. In previous papers, gelatin nanoparticles synthesized from recombinant human gelatin by one-step desolvation method were described [23], therefore, we believe that the universal nature of the proposed method enables usage of human recombinant gelatin as a starting material.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S. Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies. Polymers 2020, 12, 3051. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A. Mini-Review: Opportunities and challenges in the techniques used for preparation of gelatin nanoparticles. Pak. J. Pharm. Sci. 2020, 33, 221–228. [Google Scholar] [PubMed]
- Alipal, J.; Pu’Ad, N.M.; Lee, T.C.; Nayan, N.; Sahari, N.; Basri, H.; Idris, M.; Abdullah, H. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Miernicki, M.; Hofmann, T.; Eisenberger, I.; von der Kammer, F.; Praetorius, A. Legal and practical challenges in classifying nanomaterials according to regulatory definitions. Nat. Nanotechnol. 2019, 14, 208–216. [Google Scholar] [CrossRef]
- Kianfar, E. Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnol. 2021, 19, 159. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Yasmin, R.; Shah, M.; Khan, S.A.; Ali, R. Gelatin nanoparticles: A potential candidate for medical applications. Nanotechnol. Rev. 2017, 6, 191–207. [Google Scholar] [CrossRef]
- Esteban-Pérez, S.; Andrés-Guerrero, V.; López-Cano, J.J.; Molina-Martínez, I.; Herrero-Vanrell, R.; Bravo-Osuna, I. Gelatin Nanoparticles-HPMC Hybrid System for Effective Ocular Topical Administration of Antihypertensive Agents. Pharmaceutics 2020, 12, 306. [Google Scholar] [CrossRef] [Green Version]
- Sudheesh, M.S.; Vyas, S.P.; Kohli, D.V. Nanoparticle-based immunopotentiation via tetanus toxoid-loaded gelatin and aminated gelatin nanoparticles. Drug Deliv. 2011, 18, 320–330. [Google Scholar] [CrossRef]
- Narayanan, D.; Geena, M.G.; Lakshmi, H.; Koyakutty, M.; Nair, S.; Menon, D. Poly-(ethylene glycol) modified gelatin nanoparticles for sustained delivery of the anti-inflammatory drug Ibuprofen-Sodium: An in vitro and in vivo analysis. Nanomedicine 2013, 9, 818–828. [Google Scholar] [CrossRef]
- Klier, J.; Bartl, C.; Geuder, S.; Geh, K.J.; Reese, S.; Goehring, L.S.; Winter, G.; Gehlen, H. Immunomodulatory asthma therapy in the equine animal model: A dose-response study and evaluation of a long-term effect. Immun. Inflamm. Dis. 2019, 7, 130–149. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Liu, Y.; Zhu, X.; Chen, X.; Liu, J.; Zhou, Y.; Qin, X.; Liu, J. Bacteria-Responsive Biomimetic Selenium Nanosystem for Multidrug-Resistant Bacterial Infection Detection and Inhibition. ACS Nano 2019, 13, 13965–13984. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part B 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, C.C.; Aleman, J.; Mutkus, L.; Skardal, A. A mechanically robust thixotropic collagen and hyaluronic acid bioink supplemented with gelatin nanoparticles. Bioprinting 2019, 16, e00058. [Google Scholar] [CrossRef]
- Diba, M.; Koons, G.L.; Bedell, M.L.; Mikos, A.G. 3D printed colloidal biomaterials based on photo-reactive gelatin nanoparticles. Biomaterials 2021, 274, 120871. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Chen, K.; Wang, Z.; Huang, B.; Wang, Y.; Yu, M.; Liu, W.; Guo, S.-S.; Zhao, X.-Z. Multifunctional Gelatin Nanoparticle Integrated Microchip for Enhanced Capture, Release, and Analysis of Circulating Tumor Cells. Part. Part. Syst. Charact. 2019, 36, 1900076. [Google Scholar] [CrossRef]
- Feng, X.; Dai, H.; Ma, L.; Fu, Y.; Yu, Y.; Zhou, H.; Guo, T.; Zhu, H.; Wang, H.; Zhang, Y. Properties of Pickering emulsion stabilized by food-grade gelatin nanoparticles: Influence of the nanoparticles concentration. Colloids Surf. B 2020, 196, 111294. [Google Scholar] [CrossRef]
- Coester, C.J.; Langer, K.; van Briesen, H.; Kreuter, J. Gelatin nanoparticles by two step desolvation--a new preparation method, surface modifications and cell uptake. J. Microencapsul. 2000, 17, 187–193. [Google Scholar] [CrossRef]
- Zwiorek, K. Gelatin Nanoparticles as Delivery System for Nucleotide-Based Drugs. Ph.D. Thesis, Ludwig Maximilian University of Munich, Munich, Germany, 3 August 2006. [Google Scholar]
- Ahlers, M.; Coester, C.; Zwiorek, K.; Zillies, J. Nanoparticles and Method for the Production. Thereof. Patent WO2006021367A1, 18 August 2005. [Google Scholar]
- Geh, K.J.; Hubert, M.; Winter, G. Optimisation of one-step desolvation and scale-up of gelatine nanoparticle production. J. Microencapsul. 2016, 33, 595–604. [Google Scholar] [CrossRef]
- Won, Y.-W.; Kim, Y.-H. Recombinant human gelatin nanoparticles as a protein drug carrier. J. Control. Release 2008, 127, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Madkhali, O. Cationic Gelatin/Pluronic-based Nanoparticles as Novel Non-Viral Delivery Systems for Gene Therapy. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2018. [Google Scholar]
- Stevenson, A.T.; Lewis, S.A.; Whittington, A.R. Filtration initiated selective homogeneity (FISH) desolvation: A new method to prepare gelatin nanoparticles with high physicochemical consistency. Food Hydrocoll. 2018, 84, 337–342. [Google Scholar] [CrossRef]
- Shamarekh, K.S.; Gad, H.A.; Soliman, M.E.; Sammour, O.A. Towards the production of monodisperse gelatin nanoparticles by modified one step desolvation technique. J. Pharm. Invest. 2020, 50, 189–200. [Google Scholar] [CrossRef]
- Kaul, G.; Amiji, M. Long-circulating poly(ethylene glycol)-modified gelatin nanoparticles for intracellular delivery. Pharm. Res. 2002, 19, 1061–1067. [Google Scholar] [CrossRef]
- Kommareddy, S.; Amiji, M.M. Preparation and Loading of Gelatin Nanoparticles. Cold Spring Harb. Protoc. 2008, 2, pdb–prot4885. [Google Scholar] [CrossRef]
- Ofokansi, K.; Winter, G.; Fricker, G.; Coester, C. Matrix-loaded biodegradable gelatin nanoparticles as new approach to improve drug loading and delivery. Eur. J. Pharm. Biopharm. 2010, 76, 1–9. [Google Scholar] [CrossRef]
- Khramtsov, P.; Kalashnikova, T.; Bochkova, M.; Kropaneva, M.; Timganova, V.; Zamorina, S.; Rayev, M. Measuring the concentration of protein nanoparticles synthesized by desolvation method: Comparison of Bradford assay, BCA assay, hydrolysis/UV spectroscopy and gravimetric analysis. Int. J. Pharm. 2021, 15, 120422. [Google Scholar] [CrossRef]
- Beyer, C.; Claisen, L. Ueber die Einführung von Säureradicalen in Ketone. Ber. Dtsch. Chem. Ges. 1887, 20, 2178. [Google Scholar] [CrossRef] [Green Version]
- Mironov, L.Y.; Parfenov, P.S.; Shurukhina, A.V.; Lebedev, Y.I.; Metlenko, A.A. Delayed Fluorescence of Dyes Sensitized by Eu3+ Chelate Nanoparticles. J. Phys. Chem. C 2017, 121, 19958–19965. [Google Scholar] [CrossRef]
- De Abreu Costa, L.; Henrique Fernandes Ottoni, M.; Dos Santos, M.G.; Meireles, A.B.; Gomes de Almeida, V.; de Fátima Pereira, W.; Alves de Avelar-Freitas, B.; Eustáquio Alvim Brito-Melo, G. Dimethyl Sulfoxide (DMSO) Decreases Cell Proliferation and TNF-α, IFN-γ, and IL-2 Cytokines Production in Cultures of Peripheral Blood Lymphocytes. Molecules 2017, 22, 1789. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Kwak, M.; Lee, T.G.; Lee, J.Y. Quantifying the level of nanoparticle uptake in mammalian cells using flow cytometry. Nanoscale 2020, 12, 15743–15751. [Google Scholar] [CrossRef]
- Farrugia, C.A.; Groves, M.J. Gelatin Behaviour in Dilute Aqueous Solution: Designing a Nanoparticulate Formulation. J. Pharm. Pharmacol. 1999, 51, 643–649. [Google Scholar] [CrossRef] [Green Version]
- Vandervoort, J.; Ludwig, A. Preparation and evaluation of drug-loaded gelatin nanoparticles for topical ophthalmic use. Eur. J. Pharm. Biopharm. 2004, 57, 251–261. [Google Scholar] [CrossRef]
- Singh, A.; Xu, J.; Mattheolabakis, G.; Amiji, M. EGFR-targeted gelatin nanoparticles for systemic administration of gemcitabine in an orthotopic pancreatic cancer model. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 589–600. [Google Scholar] [CrossRef] [Green Version]
- Von Storp, B.; Engel, A.; Boeker, A.; Ploeger, M.; Langer, K. Albumin nanoparticles with predictable size by desolvation procedure. J. Microencapsul. 2012, 29, 138–146. [Google Scholar] [CrossRef]
- Matthew, S.A.L.; Totten, J.D.; Phuagkhaopong, S.; Egan, G.; Witte, K.; Perrie, Y.; Seib, F.P. Silk Nanoparticle Manufacture in Semi-Batch Format. ACS Biomater. Sci. Eng. 2020, 14, 6748–6759. [Google Scholar] [CrossRef]
- Mehravar, R.; Jahanshahi, R.; Najafpour, G.D.; Saghatoleslami, N. Applying the Taguchi method for optimized fabrication of α-lactalbumin nanoparticles as carrier in drug delivery and food science. Iran. J. Energy Environ. 2011, 2, 87–91. [Google Scholar]
- Subara, D.; Jaswir, I.; Alkhatib, M.F.R.; Noorbatcha, I.A. Synthesis of fish gelatin nanoparticles and their application for the drug delivery based on response surface methodology. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 045014. [Google Scholar] [CrossRef]
- Abdelrady, H.; Hathout, R.M.; Osman, R.; Saleem, I.; Mortada, N.D. Exploiting gelatin nanocarriers in the pulmonary delivery of methotrexate for lung cancer therapy. Eur. J. Pharm. Sci. 2019, 133, 115–126. [Google Scholar] [CrossRef]
- Pei, Y.; Zheng, Y.; Li, Z.; Liu, J.; Zheng, X.; Tang, K.; Kaplan, D.L. Ethanol-induced coacervation in aqueous gelatin solution for constructing nanospheres and networks: Morphology, dynamics and thermal sensitivity. J. Colloid Interface Sci. 2021, 582, 610–618. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.; Trouillet, V.; Clasen, A.; Jung, G.; Hollemeyer, K.; Schneider, M. NIR-Emitting Gold Nanoclusters–Modified Gelatin Nanoparticles as a Bioimaging Agent in Tissue. Adv. Healthc. Mater. 2019, 8, 1900993. [Google Scholar] [CrossRef] [PubMed]
- Subara, D.; Jaswir, I. The Effect of Processing Parameters on the Properties of Fish Gelatin Hydrolysate Nanoparticle. J. Sci. Appl. Technol. 2021, 1, 17–24. [Google Scholar] [CrossRef]
- Seib, F.P.; Jones, G.T.; Rnjak-Kovacina, J.; Lin, Y.; Kaplan, D.L. pH-dependent anticancer drug release from silk nanoparticles. Adv. Healthc. Mater. 2013, 2, 1606–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morel, M.-H.; Redl, A.; Guilbert, S. Mechanism of Heat and Shear Mediated Aggregation of Wheat Gluten Protein upon Mixing. Biomacromolecules 2002, 3, 488–497. [Google Scholar] [CrossRef]
- Balthasar, S. Charakterisierung Proteinbasierter Nanopartikel Zum Transport von Oligonukleotiden für eine Rezeptor-Vermittelte Zellaufnahme. Ph.D. Thesis, Goethe–Universität Frankfurt am Main, Frankfurt, Germany, 2005. Available online: http://d-nb.info/978388593/34 (accessed on 15 July 2021).
- Azarmi, S.; Huang, Y.; Chen, H.; McQuarrie, S.; Abrams, D.; Roa, W.; Finlay, W.H.; Miller, G.G.; Löbenberg, R. Optimization of a two-step desolvation method for preparing gelatin nanoparticles and cell uptake studies in 143B osteosarcoma cancer cells. J. Pharm. Pharm. Sci. 2006, 9, 124–132. [Google Scholar]
- Arroyo-Maya, I.J.; Rodiles-López, J.O.; Cornejo-Mazón, M.; Gutiérrez-López, G.F.; Hernández-Arana, A.; Toledo-Núñez, C.; Barbosa-Cánovas, G.V.; Flores-Flores, J.O.; Hernández-Sánchez, H. Effect of different treatments on the ability of α-lactalbumin to form nanoparticles. J. Dairy Sci. 2012, 95, 6204–6214. [Google Scholar] [CrossRef]
- Sun, S.; Xiao, Q.-R.; Wang, Y.; Jiang, Y. Roles of alcohol desolvating agents on the size control of bovine serum albumin nanoparticles in drug delivery system. J. Drug Deliv. Sci. Technol. 2018, 47, 193–199. [Google Scholar] [CrossRef]
- Mohammad-Beigi, H.; Shojaosadati, S.A.; Morshedi, D.; Mirzazadeh, N.; Arpanaei, A. The Effects of Organic Solvents on the Physicochemical Properties of Human Serum Albumin Nanoparticles. Iran. J. Biotechnol. 2016, 14, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Nixon, J.R.; Khalil, S.A.H.; Carless, J.E. Phase relationships in the simple coacervating system isoelectric gelatin: Ethanol: Water. J. Pharm. Pharmacol. 1966, 18, 409–416. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Rao, C.M. The role of surface charge in the desolvation process of gelatin: Implications in nanoparticle synthesis and modulation of drug release. Int. J. Nanomed. 2017, 12, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.; Zhang, T.; Zhang, H.; Tao, N.; Wang, X.; Zhong, J. Effect of preparation factors and storage temperature on fish oil-loaded crosslinked gelatin nanoparticle pickering emulsions in liquid forms. Food Hydrocoll. 2019, 95, 326–335. [Google Scholar] [CrossRef]
- Spoljaric, S.; Ju, Y.; Caruso, F. Protocols for Reproducible, Increased-Scale Synthesis of Engineered Particles—Bridging the “Upscaling Gap”. Chem. Mater. 2021, 33, 1099–1115. [Google Scholar] [CrossRef]
- Langer, K.; Anhorn, M.G.; Steinhauser, I.; Dreis, S.; Celebi, D.; Schrickel, N.; Faust, S.; Vogel, V. Human serum albumin (HSA) nanoparticles: Reproducibility of preparation process and kinetics of enzymatic degradation. Int. J. Pharm. 2008, 347, 109–117. [Google Scholar] [CrossRef]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Balthasar, S.; Michaelis, K.; Dinauer, N.; Von Briesen, H.; Kreuter, J.; Langer, K. Preparation and characterisation of antibody modified gelatin nanoparticles as drug carrier system for uptake in lymphocytes. Biomaterials 2005, 26, 2723–2732. [Google Scholar] [CrossRef]
- Leo, E.; Vandelli, M.A.; Cameroni, R.; Forni, F. Doxorubicin-loaded gelatin nanoparticles stabilized by glutaraldehyde: Involvement of the drug in the cross-linking process. Int. J. Pharm. 1997, 155, 75–82. [Google Scholar] [CrossRef]
- Cai, B.; Rao, L.; Ji, X.; Bu, L.L.; He, Z.; Wan, D.; Yang, Y.; Liu, W.; Guo, S.; Zhao, X.Z. Autofluorescent gelatin nanoparticles as imaging probes to monitor matrix metalloproteinase metabolism of cancer cells. J. Biomed. Mater. Res. Part A 2016, 104, 2854–2860. [Google Scholar] [CrossRef]
- Wei, W.; Wang, L.-Y.; Yuan, L.; Wei, Q.; Yang, X.-D.; Su, Z.-G.; Ma, G.-H. Preparation and Application of Novel Microspheres Possessing Autofluorescent Properties. Adv. Funct. Mater. 2007, 17, 3153–3158. [Google Scholar] [CrossRef]
- Tsai, Y.-J.; Hu, C.-C.; Chu, C.-C.; Imae, T. Intrinsically fluorescent PAMAM dendrimer as gene carrier and nanoprobe for nucleic acids delivery: Bioimaging and transfection study. Biomacromolecules 2011, 12, 4283–4290. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, M.K.; Nayak, M.K.; Kumari, S.; Grácio, J.J.A.; Dash, D. Size distribution analysis and physical/fluorescence characterization of graphene oxide sheets by flow cytometry. Carbon 2011, 49, 684–692. [Google Scholar] [CrossRef]
- Sivera, M.; Kvitek, L.; Soukupova, J.; Panacek, A.; Prucek, R.; Vecerova, R.; Zboril, R. Silver nanoparticles modified by gelatin with extraordinary pH stability and long-term antibacterial activity. PLoS ONE 2014, 9, e103675. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, S. Gelatin Nanoparticles as a Modern Platform for Drug Delivery: Formulation Development and Immunotherapeutic Strategies. Ph.D. Thesis, Ludwig Maximilian University of Munich, Munich, Germany, 29 July 2010. [Google Scholar]
- Shilpi, D.; Kushwah, V.; Agrawal, A.K.; Jain, S. Improved Stability and Enhanced Oral Bioavailability of Atorvastatin Loaded Stearic Acid Modified Gelatin Nanoparticles. Pharm. Res. 2017, 34, 1505–1516. [Google Scholar] [CrossRef]
- Geh, K. Gelatine Nanoparticles as Immunomodulatory Drug Delivery System: Advanced Production Processes and Clinical Trials. Ph.D. Thesis, Ludwig Maximilian University of Munich, Munich, Germany, 16 March 2018. [Google Scholar] [CrossRef]
- Vetten, M.A.; Yah, C.S.; Singh, T.; Gulumian, M. Challenges facing sterilization and depyrogenation of nanoparticles: Effects on structural stability and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1391–1399. [Google Scholar] [CrossRef]
- Ma, X.; Hargrove, D.; Dong, Q.; Song, D.; Chen, J.; Wang, S.; Lu, X.; Cho, Y.K.; Fan, T.-H.; Lei, Y. Novel green and red autofluorescent protein nanoparticles for cell imaging and in vivo biodegradation imaging and modeling. RSC Adv. 2016, 6, 50091–50099. [Google Scholar] [CrossRef]
- Langevin, D.; Raspaud, E.; Mariot, S.; Knyazev, A.; Stocco, A.; Salonen, A.; Luch, A.; Haase, A.; Trouiller, B.; Relier, C.; et al. Towards reproducible measurement of nanoparticle size using dynamic light scattering: Important controls and considerations. NanoImpact 2018, 10, 161–167. [Google Scholar] [CrossRef]
- Monti, F.; Manfredi, G.; Palamà, I.E.; Kovtun, A.; Zangoli, M.; D’Amone, S.; Ortolani, L.; Bondelli, G.; Szreder, T.; Bobrowski, K.; et al. Sterilization of Semiconductive Nanomaterials: The Case of Water-Suspended Poly-3-Hexylthiophene Nanoparticles. Adv. Healthc. Mater. 2021, 10, 2001306. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Hwang, J.; Lee, H.; Hammond, P.T.; Choi, J.; Hong, J. In vitro blood cell viability profiling of polymers used in molecular assembly. Sci. Rep. 2017, 7, 9481. [Google Scholar] [CrossRef]
- Ong, Y.R.; De, R.; Johnston, A.P.R. In Vivo Quantification of Nanoparticle Association with Immune Cell Subsets in Blood. Adv. Healthc. Mater. 2021, 10, 2002160. [Google Scholar] [CrossRef]
- A matter of scale. Nat. Nanotechnol. 2016, 11, 733. [CrossRef] [Green Version]
- Feng, J.; Markwalter, C.E.; Tian, C.; Armstrong, M.; Prud’homme, R.K. Translational formulation of nanoparticle therapeutics from laboratory discovery to clinical scale. J. Transl. Med. 2019, 17, 200. [Google Scholar] [CrossRef]
- Van Den Broek, S.; Nieuwland, P.J.; Kaspar Koch, K. Continuous Flow Production of Gelatin Nanoparticles. U.S. Patent US9289499B2, 22 March 2016. [Google Scholar]
- Chiesa, E.; Dorati, R.; Pisani, S.; Conti, B.; Bergamini, G.; Modena, T.; Genta, I. The Microfluidic Technique and the Manufacturing of Polysaccharide Nanoparticles. Pharmaceutics 2018, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chen, Q.; Ma, Y.; Sun, J. Microfluidic Methods for Fabrication and Engineering of Nanoparticle Drug Delivery Systems. ACS Appl. Bio. Mater. 2020, 3, 107–120. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Nanomaterials Synthesis through Microfluidic Methods: An Updated Overview. Nanomaterials 2021, 11, 864. [Google Scholar] [CrossRef]
- Varca, G.H.C.; Queiroz, R.G.; Lugão, A.B. Irradiation as an alternative route for protein crosslinking: Cosolvent free BSA nanoparticles. Radiat. Phys. Chem. 2016, 124, 111–115. [Google Scholar] [CrossRef]
- Egorova, K.S.; Galushko, A.S.; Ananikov, V.P. Introducing tox-Profiles of Chemical Reactions. Angew. Chem. Int. Ed. Engl. 2020, 59, 22296–22305. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927. [Google Scholar] [CrossRef]
- Vinjamuri, B.P.; Papachrisanthou, K.; Haware, R.V.; Chougule, M.B. Gelatin solution pH and incubation time influences the size of the nanoparticles engineered by desolvation. J. Drug Deliv. Sci. Technol. 2021, 63, 102423. [Google Scholar] [CrossRef]












| Batch ID | Suspension Volume, mL | Concentration, mg/mL | Total Dry Weight of Nanoparticles, mg 1 | Yield, % | Dh, nm 1 | PdI 2 | Zeta Potential, mV |
|---|---|---|---|---|---|---|---|
| B75-1 | 45 | 18.0 | 810.0 | 81.0 | 189 ± 8 3 | 0.014 ± 0.007 | −11.5 ± 0.6 |
| B75-2 | 46 | 17.7 | 815.6 | 81.6 | 171 ± 8 | 0.030 ± 0.012 | −10.2 ± 0.6 |
| B75-3 | 44 | 18.2 | 800.8 | 80.1 | 165 ± 4 | 0.033 ± 0.029 | −10.7 ± 0.6 |
| B225-1 | 45 | 13.8 | 621.0 | 62.1 | 133 ± 4 | 0.069 ± 0.022 | −11.5 ± 0.5 |
| B225-2 | 45 | 14.4 | 646.2 | 64.6 | 139 ± 6 | 0.094 ± 0.034 | −10.9 ± 0.3 |
| B225-3 | 45 | 15.2 | 685.4 | 68.5 | 143 ± 5 | 0.127 ± 0.065 | −11.1 ± 0.6 |
| FISH-1 | 46 | 17.8 | 817.0 | 81.7 | 164 ± 7 | 0.114 ± 0.047 | −9.0 ± 1.0 |
| FISH-2 | 46 | 16.9 | 778.8 | 77.9 | 156 ± 6 | 0.094 ± 0.052 | −9.4 ± 0.3 |
| FISH-3 | 46 | 16.9 | 775.6 | 77.6 | 151 ± 3 | 0.107 ± 0.029 | −7.1 ± 0.8 |
| A62-1 | 46 | 16.9 | 777.4 | 77.7 | 157 ± 8 | 0.054 ± 0.012 | −7.9 ± 0.9 |
| A62-2 | 45 | 16.4 | 738.0 | 73.8 | 148 ± 4 | 0.064 ± 0.012 | −7.8 ± 1.3 |
| A62-3 | 45 | 14.5 | 652.5 | 65.3 | 151 ± 4 | 0.052 ± 0.004 | −7.5 ± 0.4 |
| A180-1 | 45 | 15.8 | 711.0 | 71.1 | 144 ± 3 | 0.088 ± 0.023 | −7.1 ± 0.7 |
| A180-2 | 46 | 17.0 | 782.0 | 78.2 | 142 ± 2 | 0.060 ± 0.014 | −6.9 ± 0.7 |
| A180-3 | 45 | 17.1 | 769.5 | 77.0 | 145 ± 4 | 0.087 ± 0.036 | −7.4 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khramtsov, P.; Burdina, O.; Lazarev, S.; Novokshonova, A.; Bochkova, M.; Timganova, V.; Kiselkov, D.; Minin, A.; Zamorina, S.; Rayev, M. Modified Desolvation Method Enables Simple One-Step Synthesis of Gelatin Nanoparticles from Different Gelatin Types with Any Bloom Values. Pharmaceutics 2021, 13, 1537. https://doi.org/10.3390/pharmaceutics13101537
Khramtsov P, Burdina O, Lazarev S, Novokshonova A, Bochkova M, Timganova V, Kiselkov D, Minin A, Zamorina S, Rayev M. Modified Desolvation Method Enables Simple One-Step Synthesis of Gelatin Nanoparticles from Different Gelatin Types with Any Bloom Values. Pharmaceutics. 2021; 13(10):1537. https://doi.org/10.3390/pharmaceutics13101537
Chicago/Turabian StyleKhramtsov, Pavel, Oksana Burdina, Sergey Lazarev, Anastasia Novokshonova, Maria Bochkova, Valeria Timganova, Dmitriy Kiselkov, Artem Minin, Svetlana Zamorina, and Mikhail Rayev. 2021. "Modified Desolvation Method Enables Simple One-Step Synthesis of Gelatin Nanoparticles from Different Gelatin Types with Any Bloom Values" Pharmaceutics 13, no. 10: 1537. https://doi.org/10.3390/pharmaceutics13101537