1. Introduction
In orthopedic surgery, osteomyelitis (OM) is a dreaded complication that can affect up to 27% of patients with bone fractures [
1]. It is generally accepted that a rigorous debridement of infected bone combined with local and systemic antibiotics is necessary to achieve the highest chance of successful treatment outcome. Nonetheless, for approximately 30% of patients that undergo such extensive therapies, infection re-emerges within 12 months [
2]. Gram-positive
Staphylococcus aureus (
S. aureus) [
3] and Gram-negative
Escherichia coli (
E. coli) [
4] are two main pathogens involved in adult OM. It has been recently shown that
S. aureus has the ability to deform and penetrate (sub-)micron structures in vitro [
5] and this was also observed microscopically in clinical bone biopsies [
6]. This phenomenon is expected to contribute to the high recurrence rate of OM as bacteria residing in bone canaliculi may evade local and systemic antibiotic therapies without causing tissue necrosis and so may easily be missed during surgical debridement procedures.
The presence of bacteria deep within the bone necessitates more efficient antimicrobial delivery strategies. One approach is the direct functionalization of the antibiotic molecules with bone targeting groups, forming a bone targeting prodrug of the active antibiotic [
7,
8]. Despite straightforward synthesis strategies, there are some downsides to the prodrug approach [
9]. The prodrug must show appropriate and controlled conversion into its therapeutically active parent drug and from a translational perspective, the prodrug would need to undergo renewed approval by regulatory institutions before clinical trials can be considered.
An alternative strategy for enhanced local antibiotic delivery is to load antibiotics into carrier constructs that can release the drug payload in the vicinity of the infection site over a suitable period [
10]. For several decades, antibiotic loaded bulk materials like poly(methylmethacrylate) (PMMA) and collagen have been used as carrier materials in orthopedic surgery and have improved post-operative outcomes significantly [
11]. Due to the non-biodegradable nature of PMMA, a second surgical procedure is needed to retrieve these materials after antibiotic release. Alternatively, collagen carriers (e.g., GENTA-COLL
® and Collatamp
®G) are biodegradable, but most of these materials are characterized by their very rapid release of antibiotic load [
12]. Some other biodegradable bulk constructs can offer a more sustained release of antibiotic loads, for example poly(trimethylene carbonate) materials, and remove the need for an extraction surgery [
13,
14]. However, a common feature with all these bulk materials is the inability to localize themselves consistently at the bone interface. Antimicrobials loaded within micro or nanoparticles might offer an advantage in this regard [
15].
Several attempts have been made to design micro- and nanoparticles for sustained antibiotic delivery [
16,
17], in which delivery systems were endowed with bone affinity through incorporation of bisphosphonate groups [
18]. There are several technologies available for solid microsphere fabrication [
19,
20], which include evaporation-based methods such as electrospray [
21] or O/W emulsions and microfluidic methods or based on polymerization of polymeric emulsions [
22] for the formation of crosslinked solid microspheres. In this work, a mechanically established O/W emulsion followed by solvent evaporation was selected as a scalable and straightforward approach for the fabrication of large quantities of microspheres.
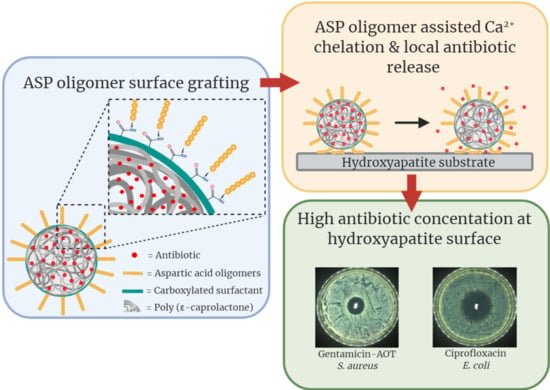
We previously reported on an antibiotic delivery system consisting of Alendronate (ALN) functionalized poly(ϵ-caprolactone) (PCL) microspheres in which hydrophobic Gentamicin-dioctyl sulfosuccinate (GM-AOT) was embedded in the PCL polymer matrix [
18]. However, ALN is known to inhibit osteoclast formation and bone mineral resorption [
23] with several ALN-related clinical side effects reported such as bisphosphonate-related osteonecrosis of the jaw (BRONJ) [
24], and so alternative bone targeting groups are preferred. Just like ALN, aspartic acid (ASP) oligomers can act as a chelator and interact with divalent ions like Ca
2+ that are present in inorganic bone matrix, but do not display any secondary effects such as those associated with bisphosphonates. Studies regarding the chelation efficacy of ASP oligomers have reported that oligomers of six or more ASP monomers show the highest affinity to bone minerals [
25]. During an investigation of estradiol-ASP prodrugs, residence time of ASP oligomers at the bone site was shown be prolonged by incorporating
d-configurated ASP monomers into the oligomers, because
d-ASP is not hydrolysable under physiological conditions in contrast to
l-ASP [
25]. ASP oligomers have been used to endow bone affinity to radionuclides [
26] and poly(lactic-co-glycolic acid) (PLGA) particles [
27]. Implementation of ASP oligomers for bone targeted antibiotic therapy has, to the best of our knowledge, not been reported yet. In this work we aimed to develop an oil/water (O/W) emulsion-based production of antibiotic loaded PCL microspheres with (
d-ASP)
6 oligomers grafted on the surface via carboxylated PVA (cPVA). Once produced, we further characterize microsphere size and antibiotic loading efficiency as well as their affinity to hydroxyapatite (HAP). Assessment of elution of two antibiotics from the microspheres and their ability to kill Gram-positive and Gram-negative planktonic bacteria and prevent bacterial growth were also studied. Finally, the advantages of ASP functionalized microspheres as antibiotic delivery systems to bone were examined in terms of establishing high local concentrations at a bone mimicking hydroxyapatite surface.
2. Materials and Methods
2.1. Materials
Poly(vinyl alcohol) (PVA; MW = 30,000 g·mol−1; 100% hydrolyzed,), poly(ϵ-caprolactone) (PCL; MW = 80,000 g·mol−1), 2-(N-morpholino)ethanesulfonic acid (MES), 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), N-hydroxysuccinimide (NHS), phosphate-buffered saline (PBS), succinic anhydride, dioctyl sulfosuccinate sodium salt (AOT), and ciprofloxacin (CF) were purchased from Sigma Aldrich, Steinheim, Germany. Gentamicin sulphate, 4-Dimethylaminopyridine (4-DMAP), ethanol, 2-propanol, acetone, and dichloromethane (DCM) were purchased from Roth (Karlsuhe, Germany). d-(Aspartic acid)6 oligomers (ASP) were synthesized on demand by GenScript (Leiden, The Netherlands). Tryptin Soy Agar (TSA) plates were purchased from Oxoid AG (Basel, Switzerland). CellTiter Blue® cell viability assay was purchased from Promega, Madison, WI, USA. Bone marrow-derived mesenchymal stem cells (BMSC) were isolated from human bone marrow donated by the Inselspital Bern. Osteoassay plates were acquired from Corning (Amsterdam, The Netherlands). The bacterial strains used were methicillin susceptible S. aureus JAR 890 (CCOS, Wädenswill, Switzerland) and E. coli O76:H51 (CCOS, Wädenswill, Switzerland).
2.2. Hydrophobic Gentamicin (GM-AOT) Synthesis
GM-AOT was synthesized as previously reported by ter Boo et al. [
14] In short, equal volumes of 0.40%
w/v gentamicin sulphate in buffer (10 mM sodium acetate, KCl and CaCl
2 at pH = 5) and 1.25%
w/v dioctyl sodium sulfosuccinate (AOT) in DCM were mixed by vigorous stirring for 3 h. The two phases dissociated for 30 min and GM-AOT was isolated from the DCM by evaporation of the solvent. Further characterization including Fourier transform infrared spectroscopy (FTIR) and the water solubility of GM-AOT was previously described [
18].
2.3. Carboxylated PVA Synthesis
The cPVA synthesis procedure was adapted from Zhang et al. [
28]. Briefly, PVA (5 g) was added to 50 mL ddH
2O and heated to 90 °C under magnetic stirring using an oil bath to allow for complete solubilization of PVA. The temperature of the PVA solution was cooled to 65 °C and 1.39 g 4-DMAP was added under moderate stirring. After 1 h, 11.36 g of succinic anhydride was added to the PVA solution and stirred at 65 °C for 24 h. The reaction was cooled to room temperature and the polymers were precipitated in 500 mL acetone and washed a second time in 200 mL acetone. The polymers were dried overnight under reduced pressure at a temperature of 40 °C. The carboxylated PVA (cPVA) product was analyzed by nuclear magnetic resonance (
1H-NMR, Bruker Avance AV 500) in D
2O solvent with a field strength of 500 MHz to assess the degree of carboxylation.
2.4. Carboxylated PVA Cytotoxicity
PVA and cPVA were dissolved ddH2O. Minimum Essential Medium (MEM) was prepared by dissolving the MEM powder in the PVA and cPVA solutions and diluting the polymer concentration with MEM. Finally, 10% Fetal Bovine Serum (FBS) and 1% penicillin/streptomycin (p/s) was added to finalize the PVA supplemented mediums. Human bone marrow derived mesenchymal stem cells (BMSC’s) were cultured in absence of PVA or cPVA in a 48-well plate with a seeding density of 5000 cells/well until a confluency of 70–80% was reached. Then, the mediums were aspirated and 400 µL of PVA supplemented medium was added to the cells (n = 6). After 24 h the PVA supplemented mediums were aspirated and a CellTiter Blue® cell viability assay was performed according to manufacturer’s instructions.
2.5. PCL/cPVA Microsphere Fabrication
A total of 500 mg PCL was dissolved in 5 mL DCM. When drug was incorporated, 125 mg of GM-AOT, CF, or pyrene was added to the PCL solution. For formulations containing CF, 1 mL of acetic acid was added as a miscible solvent in order to solubilize the antibiotic. Then, 100 mL of 1% aqueous cPVA solution that acted as a surfactant, was pH adjusted to 8.0 with 1M NaOH to ensure a favorable environment for the carboxylic acid groups of cPVA to be in a deprotonated state, in which they act as better surfactants. The polymer solution was added dropwise, under a vortex, to 10 mL of the cPVA solution over a period of approximately 1 min. This pre-emulsion was sonicated using a sonication probe (Bandelin Sonopuls GM70, Berlin, Germany) for three bursts of 30 s and finally added to the remaining 90 mL of cPVA solution. The DCM could evaporate under ambient conditions with mild magnetic stirring for 4 h. The solidified microspheres were filtered with a cell-strainer (mesh size of 70 µm) in order to remove agglomerates and washed twice in double distilled water (ddH2O) to remove cPVA that remained in solution. The PCL/cPVA microspheres were resuspended in 10 mL ddH2O, flash frozen in liquid nitrogen, and lyophilized.
2.6. PCL/cPVA Microsphere Surface Functionalization with ASP
An EDC/NHS solution was made by dissolving 100 mg EDC and 100 mg NHS in 10 mL MES-buffer at a pH of 5.5. The lyophilized PCL/cPVA microspheres (100 mg) were fully resuspended in 10 mL EDC/NHS solution by probe sonication. EDC/NHS activation of the carboxylic acid groups on the microspheres was allowed for 45 min at 4 °C. The microspheres were centrifugated and washed with ddH
2O to remove excess EDC/NHS. Next, the microspheres were dispersed in a 10 mg/mL ASP oligomer aqueous solution at neutral pH and the dispersion was stirred mildly for 2 h at room temperature. Finally, the functionalized microspheres were collected by centrifugation and washed with ddH
2O. The PCL/cPVA-ASP microspheres were flash frozen in liquid nitrogen and lyophilized. A schematic of the final microsphere product is presented in
Figure 1.
2.7. Scanning Electron Microscopy (SEM) of PCL/cPVA-ASP Microspheres
Freeze-dried PCL-cPVA-ASP microspheres loaded with GM-AOT or CF were dispersed in ethanol and 40 µL was deposited on scanning electron microscopy (SEM) specimen mounts covered with an adhesive tape. After evaporation of the ethanol, a 10 nm layer of Au/Pd was deposited on the samples by sputter-coating. SEM imaging was performed immediately after on a field emission scanning electron microscope (S-4700, Hitachi, Zürich, Switzerland) with a beam voltage of 1.0 keV. At least 100 microspheres’ diameters were measured from the SEM images using standard Axiovision4 software (version 4.9.1.0). The size polydispersity index (PDI) of the microspheres was calculated by Equation (1).
2.8. Antibiotic Encapsulation Efficiency and Antibiotic Release from PCL/cPVA-ASP Microspheres
PCL/cPVA-ASP microspheres loaded with GM-AOT or CF were disintegrated by dispersing the microspheres in 0.1M NaOH solution for several hours. Solutions containing the GM-AOT load were subsequently analyzed by complexation with o-phthaldialdehyde reagent, followed by adsorption measurement (λ = 332 nm) using a spectrophotometer (MultiskanGo, Thermo Scientific, Waltham, MA, USA) [
29]. CF encapsulation was determined by high pressure liquid chromatography (HPLC) analysis using a mobile phase of 2% Acetic acid/Acetonitrile (84:16) under a flow rate of 1 mL/min and a detection wavelength of 280 nm. To assess the drug quantity embedded inside the microspheres, the embedding efficacy (EE%) can be determined following Equation (2). The drug load (DL%) of the microspheres can be calculated with Equation (3).
With WDM as the weight of the drug embedded in the microsphere as determined by HPLC or colorimetric o-phthaldialdehyde assay, WDA as the weight of the drug added to the O/W emulsion during particle fabrication and WT as the total weight of the drug loaded microsphere sample. All EE% and DL% values were determined by averages of technical triplicates (n = 3).
To establish antibiotic release profiles, accurately weighed quantities (10 mg) of microspheres were dispersed in 1 mL PBS by probe sonication (n = 3). To allow for the collection of the complete 1 mL of supernatant, the dispersions were centrifuged with a desktop centrifuge (ROTILABO, Roth, Karlsruhe, Germany) at 9000× g after 1, 3, and 5 h and 1, 2, 3, 4, 7, 8, 9, and 13 days of incubation at 37 °C on an orbital shaker. The supernatant was replaced with 1 mL fresh PBS, followed by redispersion of the microspheres by sonication. Care was taken not to disturb the microsphere pellet during supernatant sampling. GM-AOT and CF concentrations in the collected supernatants were determined as described above.
2.9. Antimicrobial Effects on Planktonic Bacteria
The opacity of bacterial dispersions upon addition of GM-AOT or CF loaded PCL/cPVA-ASP was monitored over 48 h in order to assess the antimicrobial effect of released GM-AOT or CF. Bacterial suspensions of
S. aureus and
E. coli was initially set at an optical density at 600 nm (OD
600) of 0.5 in PBS. In triplicate, 5 mg of GM-AOT loaded microspheres were added to 1 mL of
S. aureus dispersion and 5 mg of CF loaded microspheres were added to 1 mL of
E. coli dispersion (
n = 3). The samples were gently resuspended and the OD
600 was assessed at regular intervals to assess the reduction in OD
600 caused by the bactericidal effect of the released antibiotic. Control groups for the OD measurements included bacterial dispersions of both bacterial strains without added microspheres and dispersions of GM-AOT and CF loaded microspheres in PBS (
Figure S1). For the CFU quantification, unencapsulated antibiotics in solution (0.5 mg/mL) were used as a control group (
n = 3).
2.10. Zone of Inhibition of Antibiotic Loaded PCL/cPVA-ASP Microspheres
PCL/cPVA-ASP microspheres loaded with GM-AOT or CF were dispersed in PBS at a concentration of 10 mg/mL and bacterial lawns of S. aureus or E. coli were streaked on TSA plates according to the Kirby–Bauer method. In the center of the TSA plate, 100 µL of the microsphere dispersions were carefully pipetted on a sterile blanc paper disc (Sensi-disc, BD Biosciences, Franklin Lakes, NJ, USA) in triplicate. GM-AOT loaded microspheres were tested against S. aureus bacterial lawns while CF loaded microspheres were tested on E. coli bacterial lawns. The plates were incubated overnight at 37 °C and 5% CO2 and the inhibition diameters were measured. The microsphere loaded discs were transferred to a freshly streaked TSA plate and incubated for another 24 h followed by zone of inhibition (ZOI) measurements. This process was repeated daily until no ZOI was observed.
2.11. PCL/cPVA-ASP Affinity with Hydroxyapatite Surfaces
Pyrene loaded PCL/cPVA-ASP and PCL/cPVA microspheres (5 mg/mL) were incubated in an Osteoassay 96-well plate containing thin HAP films on the bottom of the wells (n = 6). After 1 h, the wells were washed three times with ddH2O. Between each wash, the well plate was measured using a fluorescence plate reader (Viktor3, Perkin Elmer, Schwerzenbach, Switzerland) with a Hoechst filter (λex/λem = 353 nm/483 nm). A standard containing a known weight of Pyrene loaded PCL/cPVA-ASP microspheres was measured after every washing step to account for any leeching of the pyrene out of the microspheres. In order to visualize the microspheres on the HAP films, the Osteoassay 96-well plates were imaged using a fluorescent microscope (EVOS FL Auto 2, Thermo Fisher Scientific, Basel, Switzerland), again implementing the Hoechst filter settings.
2.12. Zone of Inhibition Around Hydroxyapatite Granules Incubated with PCL/cPVA-ASP
HAP granules (n = 4) with diameters between 5 and 10 mm were incubated for 1 h in a GM-AOT or CF loaded PCL/cPVA or PCL/cPVA-ASP microsphere dispersions at a concentration of 5 mg/mL under mild rocking conditions. The HAP granules were washed in PBS and were placed centrally on a TSA plate that was streaked with a S. aureus or E. coli bacterial lawn prepared as described above. The HAP granules were slightly pressed into the agar to ensure contact between the granule and the agar layer. After overnight incubation at 37 °C, the average zone of inhibition around the HAP granule was calculated from four measurements in different directions. Due to the varying sizes of the HAP granules, the distance from the granule surface to the inhibition front was measured, instead of the total diameter of the inhibition zone. The granules were transferred to a freshly streaked agar plate, in order to assess the zone of inhibition established by antibiotic released at day 2. This process was repeated daily until an inhibitory zone could no longer be observed.
2.13. Statistical Analysis
All numerical results (EE%, DL%, and microsphere diameter) are given as an average ± standard deviation (SD). Average values are presented on bar graphs and X-Y plots with the error bars representing the SD. Two sample Student’s t-tests were performed to indicate significance of results.
4. Discussion
The relatively high recurrence rate of bone infection despite appropriate therapy suggests that improvements in antibiotic delivery to the infection site are required. Antibiotic carriers currently applied in clinics possess suboptimal properties such as non-biodegradability, incomplete antibiotic release, or excessive burst release of antibiotic load. The ZOI of antibiotic loaded PMMA bone cements and collagen sheets have been reported in our previous work, showing that PMMA cements release antibiotics over 7 days while collagen materials could only establish a ZOI for 3 days [
18]. However, these frequently utilized antibiotic carriers come in bulky dimensions, necessitating invasive surgeries while preventing antibiotic release directly at the interface of the bone. In this work we introduced an antibiotic microsphere carrier system that could efficiently bind to calcified materials and release its antibiotic payload in close vicinity to infected bone mimics.
In our previous investigations, the bone targeting moiety was conjugated to COOH groups on the surface of the PCL microspheres, which were generated by alkaline hydrolysis (saponification) of PCL in 0.1M NaOH solutions. Due to hydrolytic degradation in presence of the such high pH levels, this procedure denatured the embedded GM-AOT and reduced its antimicrobial capacities. As many other antibiotics do not tolerate such extreme alkaline environments, our current motivation was to adjust the functionalization strategy by including functionalized cPVA surfactants in order to enrich the surface of the microspheres with COOH groups and omitting the harsh alkaline treatment procedure. While cPVA has been investigated for other applications (e.g., in relation with the modification of PVA crosslinking properties [
31,
32]), no reports have been found where cPVA was implemented as a surfactant for drug delivery purposes. Additionally, ASP oligomers were utilized as bone targeting moieties in this work opposed to ALN which showed impairment of osteoclast functionality in our previous work [
18] and is associated with BRONJ pathology [
24]. PCL/cPVA-ASP microspheres showed increased affinity to HAP granules compared to PCL/PVA microspheres and retained its antimicrobial properties after ASP functionalization. These results highlight the advantages of the use of cPVA surfactants instead of alkaline induced COOH formation for subsequent surface functionalization.
The investigation of drug release kinetics is essential when assessing novel drug delivery systems. For Korsmeyer–Peppas modeling of drug release, an
n-value below 0.43 correlating to drug release by means of Fickian diffusion is expected for polydisperse microsphere samples, [
33,
34] as is indeed the case in this work. The main characteristics of Fickian diffusion are defined by a slow polymer relaxation and a high diffusion rate of solvent to the interior of the microsphere. Polymers with a glass transition temperature (T
g) below the environmental temperature are often characterized by a Fickian diffusion of the drug from their polymeric matrix [
30]. As the T
g of PCL is −60 °C and the microspheres were incubated at 37 °C, this statement conforms to our findings. Polymer crystallinity is another parameter that influences drug release kinetics from polymer materials. In oil/water emulsion systems, solvent evaporation rate has been known to influence the crystalline structure of the formed microspheres [
35]. In this work, DCM was used as an organic oil phase, allowing for quick solvent evaporation and thus lower crystallization. With GM-AOT release over a 2-week period, this solvent choice and its effect on polymer crystallinity appears to be matching well with the sustained release kinetics desired for infection treatment.
Gentamicin salts (GM) and CF were used in this work as they are commonly applied in orthopedic surgery as a prophylactic agent or as a treatment for infections [
36,
37]. GM and CF are both active against Gram-positive and Gram-negative bacteria and can be considered broad-spectrum antibiotics. As GM is a hydrophilic antibiotic which is challenging to embed in polymer microspheres fabricated by O/W emulsions, we have previously applied a hydrophobic ion pairing method to substitute the sulphate ions of GM with a hydrophobic dioctyl sulfosuccinate counter-ion, resulting in the synthesis of water-insoluble GM-AOT [
14,
18]. This modified antibiotic could be efficiently embedded in hydrophobic matrices and showed similar antimicrobial properties compared to GM [
18]. Additionally, the antibiotic CF was also interesting for investigation in drug delivery to bone as its molecular structure allows for chelation to Ca
2+ [
38] and can thus potentially interact with bone after being released from the microspheres. This interaction between CF and HAP was shown to be reversible at 37 °C and CF release from HAP composite materials was monitored in previous studies [
39]. This characteristic of CF can potentially prolong the residence time of the antibiotic in bone tissue.
Even though both GM-AOT and CF loaded microspheres showed bactericidal properties (
Figure 6), a clear difference in EE% and DL% of GM-AOT and CF was observed. Lower CF loading into the microspheres could be due to the lower solubility of CF in the DCM/acetic acid co-solvent system and different miscibility properties in the subsequent emulsification within the aqueous phase. To optimize CF encapsulation a W
1/O/W
2 emulsion could be considered, in which the CF load could be incorporated into the inner aqueous phase consisting of acidic solvents such as diluted acetic acid.
Comparative literature on drug delivery through peptide-based bone targeting is limited. Jiang et al. has performed a promising study on PLGA-PEG nanoparticles (NPs) functionalized with ASP and glycine (GLY) oligomers of six repeating units [
27]. The oligomers were labeled with fluorescein isothiocyanate (FITC) in order to quantify the NPs binding affinity. Dispersions of ASP functionalized NPs were exposed to HAP containing hydrogels and NPs remaining in dispersion were quantified by light absorption measurements. Results showed that 85% (85 µg) of ASP functionalized NPs and 32% (32 µg) of GLY functionalized control NPs attached to the HA-hydrogel. This ratio between non-specific interaction of the GLY functionalized NPs and targeted interaction of the ASP functionalized NPs (1:2.65 ratio) is comparable to the results shown in
Figure 7, where 103 µg of PCL/cPVA and 346 µg of PCL/cPVA-ASP (1:3.36 ratio) was quantified on HAP surfaces. As no information of the ASP configuration (
d– or
l–) was given by Jiang et al. it could be argued that the higher affinity ratio to calcified materials that we reported could be due to the non-hydrolysable D-ASP. The interaction between HAP granules and non-functionalized PCL/cPVA microspheres could originate from a combination of non-specific adsorption and from the carboxylated surface of the microspheres. Nap et al. investigated the complex formation between two carboxylate ions and ionic calcium [
40], however, the complexation of COOH groups on the microspheres surface with Ca
2+ ions in HAP crystals is not expected to significantly contribute to the interaction between the carboxyl-rich microspheres and HAP granules.
In a final experiment, the positive effect of the bone-binding surface was demonstrated via the larger and long-lasting inhibitory zone measurements of antibiotic loaded PCL/cPVA-ASP microspheres in comparison to PCL/cPVA microspheres loaded on HAP granules. Interestingly, even though CF encapsulation in PCL/cPVA-ASP microspheres (EE% = 12.76% ± 1.78%) was lower than the encapsulation of GM-AOT (EE% = 23.77% ± 1.39%), the ZOI that resulted from CF release was higher and persisted longer. This observation can be explained due to variability in sensitivity of
S. aureus and
E. coli bacterial strains against the two antibiotics. The minimal inhibitory concentration (MIC) of GM-AOT to the used
S. aureus JAR strain was determined to be 0.98 µM [
18], while the MIC of wild type
E. coli is generally below 0.18 µM [
41]. This difference in susceptibility could be the cause of the higher efficacy of CF loaded microspheres compared to those loaded with GM-AOT. Additionally, the molecular structure of CF allows for chelation through its carboxylic acid and ketone groups [
42]. The release of chelated CF from a HAP/PCL nanocomposite substrate was previously reported and large quantities of CF (reported in absorbance values, but not correlated to a CF dosage) were detected in the release media over the course of 4 days at 37 °C [
39]. Thus, it can be hypothesized that the CF intrinsic chelating ability to HAP combined with the sustained CF release from the PCL/cPVA-ASP microspheres bonded to the HAP, further enhance the local concentration of antibiotic at the bone-like material surface.
This work reports on a new approach towards carboxylation of microparticulate systems, implementing functionalized cPVA surfactants that facilitate further grafting of functional surface moieties. We believe that this strategy is of particular interest to a multitude of applications in targeted therapeutics. Specifically, the PCL/cPVA-ASP microspheres introduced in this work show to be a versatile drug delivery platform for antibiotic delivery to bone that, once further assessed in pre-clinical infection models, is open for clinical translation.