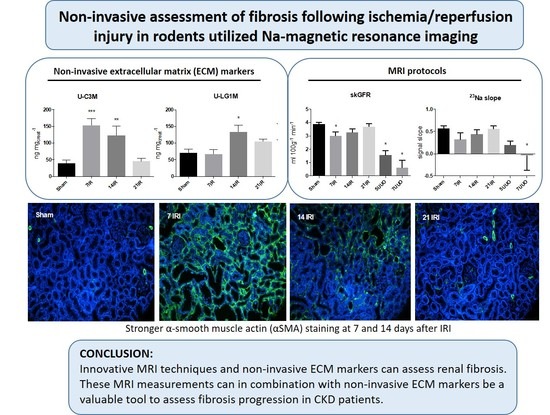
Noninvasive Assessment of Fibrosis Following Ischemia/Reperfusion Injury in Rodents Utilizing Na Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Models
2.2. Fumarase Activity Assay
2.3. Western Blotting (WB)
2.4. Histology, Immunofluorescence (IF), and Immunohistochemistry (IHC)
2.5. RNA Extraction and Quantitative PCR (qPCR)
2.6. MR Scanning
2.7. MRI Analysis
2.8. Noninvasive ECM Markers
2.9. Statistics
3. Results
3.1. Bilateral Ischemia Reperfusion Injury (IRI) and UUO Induced Kidney Injury
3.2. Bilateral IRI and UUO Induced Renal Fibrosis
3.3. Changes in the Level of ECM Markers C3M and LG1M in Response to Bilateral IRI
3.4. MRI Images, Values, and Correlations with Fibrosis Markers and Noninvasive ECM Markers
4. Discussion
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Eddy, A.A. Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int. Suppl. 2014, 4, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Leung, G.; Kirpalani, A.; Szeto, S.G.; Deeb, M.; Foltz, W.; Simmons, C.A.; Yuen, D.A. Could mri be used to image kidney fibrosis? A review of recent advances and remaining barriers. Clin. J. Am. Soc. Nephrol. 2017, 12, 1019–1028. [Google Scholar] [CrossRef]
- Morrell, G.R.; Zhang, J.L.; Lee, V.S. Magnetic resonance imaging of the fibrotic kidney. J. Am. Soc. Nephrol. 2017, 28, 2564–2570. [Google Scholar] [CrossRef]
- Friedli, I.; Crowe, L.A.; Berchtold, L.; Moll, S.; Hadaya, K.; De Perrot, T.; Vesin, C.; Martin, P.Y.; De Seigneux, S.; Vallée, J.P. New magnetic resonance imaging index for renal fibrosis assessment: A comparison between diffusion-weighted imaging and T1 mapping with histological validation. Sci. Rep. 2016, 6, 30088. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.M.; Mariager, C.Ø.; Mølmer, M.; Sparding, N.; Genovese, F.; Karsdal, M.A.; Nørregaard, R.; Bertelsen, L.B.; Laustsen, C. Hyperpolarized [1–13c] alanine production: A novel imaging biomarker of renal fibrosis. Magn. Reson. Med. 2020, 84, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Sigmund, E.E.; Chandarana, H.; Rusinek, H.; Chen, Q.; Vivier, P.H.; Taouli, B.; Lee, V.S. Variability of renal apparent diffusion coefficients: Limitations of the monoexponential model for diffusion quantification. Radiology 2010, 254, 783–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bihan, D.; Poupon, C.; Amadon, A.; Lethimonnier, F. Artifacts and pitfalls in diffusion MRI. J. Magn. Reson. Imaging 2006, 24, 478–488. [Google Scholar] [CrossRef]
- Perea, R.J.; Ortiz-Perez, J.T.; Sole, M.; Cibeira, M.T.; De Caralt, T.M.; Prat-Gonzalez, S.; Bosch, X.; Berruezo, A.; Sanchez, M.; Blade, J. T1 mapping: Characterisation of myocardial interstitial space. Insights Imaging 2015, 6, 189–202. [Google Scholar] [CrossRef] [Green Version]
- Fine, L.G.; Norman, J.T. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: From hypothesis to novel therapeutics. Kidney Int. 2008, 74, 867–872. [Google Scholar] [CrossRef] [Green Version]
- Brezis, M.; Agmon, Y.; Epstein, F.H. Determinants of intrarenal oxygenation. I. effects of diuretics. Am. J. Physiol.-Renal Physiol. 1994, 267, F1059–F1062. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Dissing, T.H.; Mørkenborg, J.; Stødkilde-Jørgensen, H.; Hansen, L.H.; Pedersen, L.B.; Grenier, N.; Frøkiær, J. Validation of quantitative bold MRI measurements in kidney: Application to unilateral ureteral obstruction. Kidney Int. 2005, 67, 2305–2312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.U.; Glockner, J.F.; Glaser, K.J.; Yin, M.; Chen, J.; Kawashima, A.; Kim, B.; Kremers, W.K.; Ehman, R.L.; Gloor, J.M. MR elastography in renal transplant patients and correlation with renal allograft biopsy: A feasibility study. Acad. Radiol. 2012, 19, 834–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, K.; Tang, H.; Mishra, P.K.; Macura, S.I.; Lerman, L.O. Measurement of murine single-kidney glomerular filtration rate using dynamic contrast-enhanced MRI. Magn. Reson. Med. 2018, 79, 2935–2943. [Google Scholar] [CrossRef]
- Andrassy, K.M. Comments on ‘Kdigo 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int. 2013, 84, 622–623. [Google Scholar] [CrossRef] [Green Version]
- Kopp, C.; Beyer, C.; Linz, P.; Dahlmann, A.; Hammon, M.; Jantsch, J.; Neubert, P.; Rosenhauer, D.; Müller, D.N.; Cavallaro, A.; et al. Na+ deposition in the fibrotic skin of systemic sclerosis patients detected by 23Na-magnetic resonance imaging. Rheumatology 2017, 56, 556–560. [Google Scholar] [CrossRef] [Green Version]
- Papasotiriou, M.; Genovese, F.; Klinkhammer, B.M.; Kunter, U.; Nielsen, S.H.; Karsdal, M.A.; Floege, J.; Boor, P. Serum and urine markers of collagen degradation reflect renal fibrosis in experimental kidney diseases. Nephrol. Dial. Transplant. 2015, 30, 1112–1121. [Google Scholar] [CrossRef] [Green Version]
- Eddy, A.A. Molecular insights into renal interstitial fibrosis. J. Am. Soc. Nephrol. 1996, 7, 2495–2508. [Google Scholar]
- Catania, J.M.; Chen, G.; Parrish, A.R. Role of matrix metalloproteinases in renal pathophysiologies. Am. J. Physiol.-Renal Physiol. 2007, 292, F905–F911. [Google Scholar] [CrossRef]
- Hijmans, R.S.; Rasmussen, D.G.; Yazdani, S.; Navis, G.; Van Goor, H.; Karsdal, M.A.; Genovese, F.; Van den Born, J. Urinary collagen degradation products as early markers of progressive renal fibrosis. J. Transl. Med. 2017, 15, 63. [Google Scholar] [CrossRef] [Green Version]
- Chawla, L.S.; Kimmel, P.L. Acute kidney injury and chronic kidney disease: An integrated clinical syndrome. Kidney Int. 2012, 82, 516–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, D.G.K.; Nielsen, P.M.; Kasab-Oglo, Ö.Y.; Nielsen, S.H.; Kierulf-Lassen, C.; Karsdal, M.A.; Genovese, F.; Nørregaard, R. A non-invasive biomarker of type III collagen degradation reflects ischaemia reperfusion injury in rats. Nephrol. Dial. Transplant. 2019, 34, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Gürtler, A.; Kunz, N.; Gomolka, M.; Hornhardt, S.; Friedl, A.A.; McDonald, K.; Kohn, J.E.; Posch, A. Stain-free technology as a normalization tool in western blot analysis. Anal. Biochem. 2013, 433, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Milani, B.; Ansaloni, A.; Sousa-Guimaraes, S.; Vakilzadeh, N.; Piskunowicz, M.; Vogt, B.; Stuber, M.; Burnier, M.; Pruijm, M. Reduction of cortical oxygenation in chronic kidney disease: Evidence obtained with a new analysis method of blood oxygenation level-dependent magnetic resonance imaging. Nephrol. Dial. Transplant. 2017, 32, 2097–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, P.M.; Eldirdiri, A.; Bertelsen, L.B.; Jørgensen, H.S.; Ardenkjaer-Larsen, J.H.; Laustsen, C. Fumarase activity: An in vivo and in vitro biomarker for acute kidney injury. Sci. Rep. 2017, 7, 40812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, F.A.; Kettunen, M.I.; Hu, D.E.; Jensen, P.R.; Zandt, R.I.; Karlsson, M.; Gisselsson, A.; Nelson, S.K.; Witney, T.H.; Bohndiek, S.E.; et al. Production of hyperpolarized [1,4-13C2]Malate from [1,4-13C2]Fumarate is a marker of cell necrosis and treatment response in tumors. Proc. Natl. Acad. Sci. USA 2009, 106, 19801–19806. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, A.; Pires, M.J.; Oliveira, P.A. Pathophysiological mechanisms of renal fibrosis: A review of animal models and therapeutic strategies. In Vivo 2017, 31, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Skrypnyk, N.I.; Harris, R.C.; De Caestecker, M.P. Ischemia-reperfusion model of acute kidney injury and post injury fibrosis in mice. J. Vis. Exp. 2013, 78, e50495. [Google Scholar] [CrossRef]
- Genovese, F.; Manresa, A.A.; Leeming, D.J.; Karsdal, M.A.; Boor, P. The extracellular matrix in the kidney: A source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenes. Tissue Repair. 2014, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Wang, F.; Quarles, C.C. Current MRI techniques for the assessment of renal disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Dekkers, I.A.; Boer, A.D.; Sharma, K.; Cox, E.F.; Lamb, H.J.; Buckley, D.L.; Bane, O.; Morris, D.M.; Prasad, P.V.; Semple, S.I.K.; et al. Consensus-based technical recommendations for clinical translation of renal T1 and T2 mapping MRI. MAGMA 2020, 33, 163–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljimani, A.; Caroli, A.; Laustsen, C.; Francis, S.; Mendichovszky, I.A.; Bane, O.; Nery, F.; Sharma, A.; Pohlmann, A.; Dekkers, I.A.; et al. Consensus-based technical recommendations for clinical translation of renal diffusion-weigthed MRI. MAGMA 2020, 33, 177–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nery, F.; Buchanan, C.E.; Harteveld, A.A.; Odudu, A.; Bane, O.; Cox, E.F.; Derlin, K.; Gach, H.M.; Golay, X.; Gutberlet, M.; et al. Consensus-based technical recommendations for clinical translation of renal ASL MRI. MAGMA 2020, 33, 141–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendichovszky, I.A.; Pullens, P.; Dekkers, I.A.; Nery, F.; Bane, O.; Pohlmann, A.; Boer, A.D.; Ljimani, A.; Odudu, A.; Buchanan, C.; et al. Technical recommendations for clinical translation of renal MRI: A consensus project of the Cooperation in Science and Technology Action PARENCHIMA. MAGMA 2020, 33, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Bane, O.; Mendichovszky, I.A.; Milani, B.; Dekkers, I.A.; Deux, J.-F.; Eckerbom, P.; Greiner, N.; Hall, M.E.; Inoue, T.; Laustsen, C.; et al. Consensus-based technical recommendations for clinical translation of renal BOLD MRI. MAGMA 2020, 33, 199–215. [Google Scholar] [CrossRef] [Green Version]
- Hockings, P.; Laustsen, C.; Joles, J.A.; Mark, P.B.; Sourbron, S. Special issue on magnetic resonance imaging biomarkers of renal diseases. MAGMA 2020, 33, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Grist, J.T.; Riemer, F.; Hansen, E.; Tougaard, R.S.; McLean, M.A.; Kaggie, J.; Bøgh, N.; Graves, M.J.; Gallagher, F.A.; Laustsen, C. Visualization of sodium dynamics in the kidney by magnetic resonance imaging by a multisite study. Kidney Int. 2020. [Google Scholar] [CrossRef]
- Pedersen, M.; Ursprung, S.; Jensen, J.D.; Jespersen, B.; Gallagher, F.; Laustsen, C. Hyperpolarised 13C-MRI metabolic and functional imaging: An emerging renal MR diagnostic modality. MAGMA 2020, 33, 23–33. [Google Scholar] [CrossRef]








| Primary Antibodies | |||
| Protein | Dilution | Company | Catalogue No. |
| α-SMA | 1:1000 | DAKO, Glostrup, Denmark | M0851 |
| Fibronectin (FN) | 1:1000 | Abcam, Cambridge, United Kingdom | ab2113 |
| Secondary Antibodies | |||
| Antigen | Company | Catalogue No. | |
| Goat anti-mouse immunoglobulin/HRP | DAKO, Glostrup, Denmark | P447 | |
| Goat anti-rabbit immunoglobulin/HRP | DAKO, Glostrup, Denmark | P448 | |
| Target Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| 18s | 5′ -CAT GGC CGT TCT TAG TTG-3′ | 5′-CAT GCC AGA GTC TCG TTC-3′ |
| α-SMA | 5′-CAT CAT GCG TCT GGA CTT GG-3′ | 5′-CCA GGG AAG AAG AGG AAG CA-3′ |
| Fibronectin | 5′-CCG AAT CAC AGT AGT TGC GG-3′ | 5′-GCA TAG TGT CCG GAC CGA TA-3′ |
| KIM-1 | 5′-CCA CAA GGC CCA CAA CTA TT-3′ | 5′-TGT CAC AGT GCC ATT CCA GT-3′ |
| NGAL | 5′-GAT CAG AAC ATT CGT TCC AA-3′ | 5′-TTG CAC ATC GTA GCT CTG TA-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, P.M.; Mariager, C.Ø.; Rasmussen, D.G.K.; Mølmer, M.; Genovese, F.; Karsdal, M.A.; Laustsen, C.; Nørregaard, R. Noninvasive Assessment of Fibrosis Following Ischemia/Reperfusion Injury in Rodents Utilizing Na Magnetic Resonance Imaging. Pharmaceutics 2020, 12, 775. https://doi.org/10.3390/pharmaceutics12080775
Nielsen PM, Mariager CØ, Rasmussen DGK, Mølmer M, Genovese F, Karsdal MA, Laustsen C, Nørregaard R. Noninvasive Assessment of Fibrosis Following Ischemia/Reperfusion Injury in Rodents Utilizing Na Magnetic Resonance Imaging. Pharmaceutics. 2020; 12(8):775. https://doi.org/10.3390/pharmaceutics12080775
Chicago/Turabian StyleNielsen, Per Mose, Christian Østergaard Mariager, Daniel Guldager Kring Rasmussen, Marie Mølmer, Federica Genovese, Morten Asser Karsdal, Christoffer Laustsen, and Rikke Nørregaard. 2020. "Noninvasive Assessment of Fibrosis Following Ischemia/Reperfusion Injury in Rodents Utilizing Na Magnetic Resonance Imaging" Pharmaceutics 12, no. 8: 775. https://doi.org/10.3390/pharmaceutics12080775