Hot-Melt 3D Extrusion for the Fabrication of Customizable Modified-Release Solid Dosage Forms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
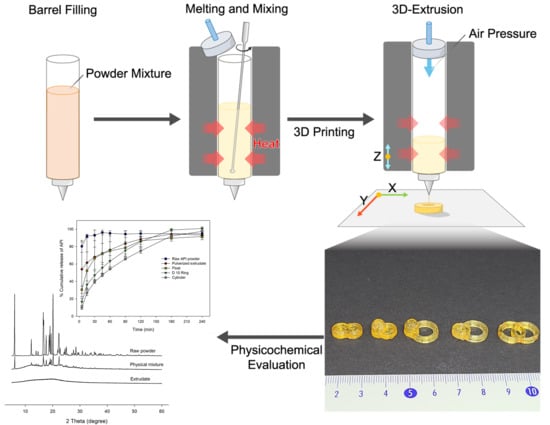
2.2. Formulation and Printing Process
2.3. Dissolution Test
2.4. API Content Test
2.5. Chromatographic Condition
2.6. Physical Parameters Measurement
2.7. Floating Test
2.8. X-Ray Diffraction
2.9. Surface Morphology
2.10. Thermal Analysis
2.11. Statistical Analysis
3. Results
3.1. Physical Properties
3.2. Solid-State of API
3.3. Thermal Analysis
3.4. Dose Adjustment
3.5. Floating Test
3.6. Dissolution Profile
3.6.1. Dissolution Profile in Acidic Condition
3.6.2. Dissolution Profile in pH 6.8 Phosphate Buffer
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shastry, B.S. Pharmacogenetics and the concept of individualized medicine. Pharm. J. 2006, 6, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, Y.; Shin, I.; Ham, G.; Abuzar, S.M.; Hyun, S.M.; Hwang, S.J. The advent of a novel manufacturing technology in pharmaceutics: Superiority of fused deposition modeling 3D printer. J. Pharm. Investig. 2020, 50, 131–145. [Google Scholar] [CrossRef]
- Wening, K.; Breitkreutz, J. Oral drug delivery in personalized medicine: Unmet needs and novel approaches. Int. J. Pharm. 2011, 404, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Choi, H.J.; Moon, S.J.; Kim, S.J.; Bajracharya, R.; Min, J.Y.; Han, H.K. Pharmaceutical applications of 3D printing technology: Current understanding and future perspectives. J. Pharm. Investig. 2019, 49, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital light processing (DLP)3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef]
- Cader, H.K.; Rance, G.A.; Alexander, M.R.; Gonçalves, A.D.; Roberts, C.J.; Tuck, C.J.; Wildman, R.D. Water-based 3D inkjet printing of an oral pharmaceutical dosage form. Int. J. Pharm. 2019, 564, 359–368. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Khaled, S.A.; Alexander, M.R.; Irvine, D.J.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. Extrusion 3D Printing of Paracetamol Tablets from a Single Formulation with Tunable Release Profiles Through Control of Tablet Geometry. AAPS PharmSciTech 2018, 19, 3403–3413. [Google Scholar] [CrossRef] [Green Version]
- Li Chew, S.; de Mohac, L.M.; Raimi-Abraham, B.T. 3D-Printed Solid Dispersion Drug Products. Pharmaceutics 2019, 11, 672. [Google Scholar] [CrossRef] [Green Version]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Fukushige, K.; Ogawa, E.; Hayashi, N.; Ozeki, T. 3D printing factors important for the fabrication of polyvinylalcohol filament-based tablets. Biol. Pharm. Bull. 2017, 40, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehtezazi, T.; Algellay, M.; Islam, Y.; Roberts, M.; Dempster, N.M.; Sarker, S.D. The Application of 3D Printing in the Formulation of Multilayered Fast Dissolving Oral Films. J. Pharm. Sci. 2018, 107, 1076–1085. [Google Scholar] [CrossRef]
- Tagami, T.; Hayashi, N.; Sakai, N.; Ozeki, T. 3D printing of unique water-soluble polymer-based suppository shell for controlled drug release. Int. J. Pharm. 2019, 568, 118494. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Nagata, N.; Hayashi, N.; Ogawa, E.; Fukushige, K.; Sakai, N.; Ozeki, T. Defined drug release from 3D-printed composite tablets consisting of drug-loaded polyvinylalcohol and a water-soluble or water-insoluble polymer filler. Int. J. Pharm. 2018, 543, 361–367. [Google Scholar] [CrossRef]
- Solanki, N.G.; Tahsin, M.; Shah, A.V.; Serajuddin, A.T.M. Formulation of 3D Printed Tablet for Rapid Drug Release by Fused Deposition Modeling: Screening Polymers for Drug Release, Drug-Polymer Miscibility and Printability. J. Pharm. Sci. 2018, 107, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Kempin, W.; Domsta, V.; Grathoff, G.; Brecht, I.; Semmling, B.; Tillmann, S.; Weitschies, W.; Seidlitz, A. Immediate Release 3D-Printed Tablets Produced Via Fused Deposition Modeling of a Thermo-Sensitive Drug. Pharm. Res. 2018, 35, 124. [Google Scholar] [CrossRef]
- Giri, B.R.; Song, E.S.; Kwon, J.; Lee, J.H.; Park, J.B.; Kim, D.W. Fabrication of intragastric floating, controlled release 3D printed theophylline tablets using hot-melt extrusion and fused deposition modeling. Pharmaceutics 2020, 12, 77. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced pharmaceutical applications of hot-melt extrusion coupled with fused deposition modelling (FDM) 3D printing for personalised drug delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef] [Green Version]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for extrusion-based 3D printing of pharmaceuticals: A holistic materials–process perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- Lakshman, J.P.; Cao, Y.; Kowalski, J.; Serajuddin, A.T.M. Application of melt extrusion in the development of a physically and chemically stable high-energy amorphous solid dispersion of a poorly water-soluble drug. Mol. Pharm. 2008, 5, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- De Brabander, C.; Van Den Mooter, G.; Vervaet, C.; Remon, J.P. Characterization of ibuprofen as a nontraditional plasticizer of ethyl cellulose. J. Pharm. Sci. 2002, 91, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Kolter, K.; Karl, M.; Gryczke, A. Hot-Melt Extrusion with BASF Pharma Polymers, Extrusion Compendium; BASF SE Pharma Ingredients & Services: Ludwigshafen, Germany, 2012. [Google Scholar]
- Akter, F.; Saha, M.; Ansary, J.; Debnath, A.; Shams, T. Study of Dissolution Characteristics of Ibuprofen by Different Polymers and Solid Dispersion Techniques. Int. J. Pharm. Sci. Res. 2015, 6, 1528–1537. [Google Scholar] [CrossRef]
- Jain, S.K.; Shukla, M.; Shrivastava, V. Development and in vitro evaluation of ibuprofen mouth dissolving tablets using solid dispersion technique. Chem. Pharm. Bull. 2010, 58, 1037–1042. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Leyva, J.C.; García-Flores, M.; Valladares-Méndez, A.; Orozco-Castellanos, L.M.; Martínez-Alfaro, M. Comparative studies on the dissolution profiles of oral ibuprofen suspension and commercial tablets using biopharmaceutical classification system criteria. Indian J. Pharm. Sci. 2012, 74, 312. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.A. The concept of dissolution efficiency. J. Pharm. Pharmacol. 1975, 27, 48–49. [Google Scholar] [CrossRef]
- Tye, C.K.; Sun, C.; Amidon, G.E. Evaluation of the effects of tableting speed on the relationships between compaction pressure, tablet tensile strength, and tablet solid fraction. J. Pharm. Sci. 2005, 94, 465–472. [Google Scholar] [CrossRef]
- Huanbutta, K.; Limmatvapirat, S.; Sungthongjeen, S.; Sriamornsak, P. Novel Strategy to Fabricate Floating Drug Delivery System Based on Sublimation Technique. AAPS PharmSciTech 2016, 17, 693–699. [Google Scholar] [CrossRef] [Green Version]
- Tita, B.; Fuliaş, A.; Bandur, G.; Rusu, G.; Tita, D. Thermal stability of ibuprofen. kinetic study under non-isothermal conditions. Rev. Roum. Chim. 2010, 55, 553–558. [Google Scholar]
- Xu, F.; Sun, L.X.; Tan, Z.C.; Liang, J.G.; Li, R.L. Thermodynamic study of ibuprofen by adiabatic calorimetry and thermal analysis. Thermochim. Acta 2004, 412, 33–57. [Google Scholar] [CrossRef]
- Phaechamud, T.; Tuntarawongsa, S. Transformation of eutectic emulsion to nanosuspension fabricating with solvent evaporation and ultrasonication technique. Int. J. Nanomed. 2016, 11, 2855–2865. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Bedi, N.; Tiwary, A.K. Enhancing solubility of poorly aqueous soluble drugs: Critical appraisal of techniques. J. Pharm. Investig. 2018, 48, 509–526. [Google Scholar] [CrossRef]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, U.M.; Selmin, F.; Ortenzi, M.A.; Mohammed, G.K.; Franzé, S.; Minghetti, P.; Cilurzo, F. Personalized orodispersible films by hot melt ram extrusion 3D printing. Int. J. Pharm. 2018, 551, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Cristofoletti, R.; Dressman, J.B. Dissolution methods to increasing discriminatory power of in vitro dissolution testing for ibuprofen free acid and its salts. J. Pharm. Sci. 2017, 106, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyanes, A.; Robles Martinez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef] [PubMed]












| Shape | Weight (mg) | Surface Area (mm2) | Volume (mm3) | SA/V | Apparent Density (g/cm3) |
|---|---|---|---|---|---|
| Cylinder | 163.33 ± 0.49 | 188.11 ± 0.21 | 159.20 ± 0.44 | 1.18 ± 0.00 | 1.03 ± 0.00 |
| D10 Ring | 162.00 ± 3.99 | 263.57 ± 5.29 | 156.96 ± 3.87 | 1.68 ± 0.01 | 1.03 ± 0.00 |
| D12 Ring | 172.37 ± 3.86 | 322.65 ± 5.89 | 168.28 ± 3.77 | 1.92 ± 0.01 | 1.02 ± 0.00 |
| D14 Ring | 182.17 ± 1.96 | 377.20 ± 3.31 | 176.47 ± 1.90 | 2.14 ± 0.01 | 1.03 ± 0.00 |
| Float | 187.53 ± 3.13 | 227.16 ± 3.08 | 287.06 ± 5.84 | 0.79 ± 0.01 | 0.65 ± 0.00 |
| CC | 274.97 ± 2.61 | 311.05 ± 2.41 | 267.91 ± 2.54 | 1.16 ± 0.00 | 1.03 ± 0.00 |
| CF | 312.50 ± 10.50 | 345.05 ± 9.48 | 396.51 ± 16.32 | 0.87 ± 0.01 | 0.79 ± 0.00 |
| CR | 275.93 ± 8.75 | 411.60 ± 10.63 | 268.62 ± 8.52 | 1.53 ± 0.02 | 1.03 ± 0.00 |
| RF | 321.50 ± 1.51 | 470.34 ± 1.81 | 403.90 ± 2.33 | 1.16 ± 0.00 | 0.80 ± 0.00 |
| RR | 339.10 ± 7.01 | 603.56 ± 10.21 | 327.99 ± 6.78 | 1.84 ± 0.01 | 1.03 ± 0.00 |
| Dosage Forms | % DE5 | % DE30 | % DE90 | % DE180 |
|---|---|---|---|---|
| Raw API powder | 40.2 ± 3.2 | 81.7 ± 2.3 | 90.6 ± 2.3 | 92.7 ± 2.5 |
| Pulverized extrudate | 27.0 ± 14.4 | 56.1 ± 24.2 | 69.4 ± 20.1 | 79.6 ± 13.6 |
| Cylinder | 5.0 ± 1.0 | 23.6 ± 2.0 | 43.6 ± 1.5 | 61.4 ± 1.4 |
| D 10 Ring | 8.3 ± 1.9 | 31.0 ± 3.7 | 51.9 ± 5.0 | 70.2 ± 4.6 |
| D 12 Ring | 10.3 ± 1.4 | 43.2 ± 8.5 | 61.1 ± 8.7 | 72.9 ± 6.2 |
| D 14 Ring | 14.3 ± 4.5 | 37.7 ± 7.7 | 57.9 ± 5.4 | 74.1 ± 3.0 |
| Float | 15.3 ± 1.9 | 46.1 ± 11.4 | 64.7 ± 9.8 | 75.5 ± 6.0 |
| CC | 5.8 ± 0.5 | 25.4 ± 1.9 | 42.4 ± 2.2 | 56.6 ± 1.9 |
| CR | 8.7 ± 0.9 | 27.5 ± 2.5 | 44.1 ± 2.7 | 59.3 ± 2.5 |
| RR | 11.3 ± 1.2 | 33.8 ± 2.8 | 50.2 ± 3.6 | 64.8 ± 3.7 |
| CF | 9.1 ± 0.8 | 30.3 ± 2.3 | 46.9 ± 2.5 | 62.2 ± 2.0 |
| RF | 10.4 ± 1.5 | 32.0 ± 2.1 | 49.2 ± 1.9 | 64.9 ± 1.8. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Song, C.; Noh, I.; Song, S.; Rhee, Y.-S. Hot-Melt 3D Extrusion for the Fabrication of Customizable Modified-Release Solid Dosage Forms. Pharmaceutics 2020, 12, 738. https://doi.org/10.3390/pharmaceutics12080738
Lee J, Song C, Noh I, Song S, Rhee Y-S. Hot-Melt 3D Extrusion for the Fabrication of Customizable Modified-Release Solid Dosage Forms. Pharmaceutics. 2020; 12(8):738. https://doi.org/10.3390/pharmaceutics12080738
Chicago/Turabian StyleLee, Jaemin, Chanwoo Song, Inhwan Noh, Sangbyeong Song, and Yun-Seok Rhee. 2020. "Hot-Melt 3D Extrusion for the Fabrication of Customizable Modified-Release Solid Dosage Forms" Pharmaceutics 12, no. 8: 738. https://doi.org/10.3390/pharmaceutics12080738