Antioxidant and Antibacterial Properties of Carbosilane Dendrimers Functionalized with Polyphenolic Moieties
Abstract
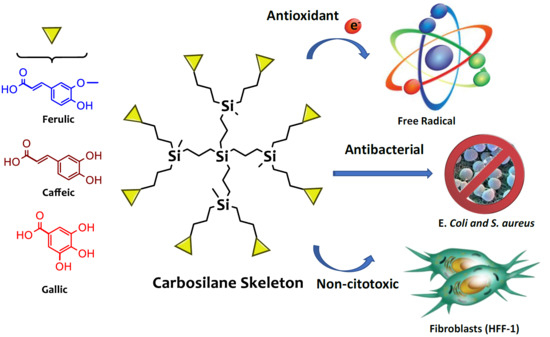
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Polyphenolic Dendrimers (1–6)
2.1.1. Synthesis of G1-[Si(CH2)3NH(CO)CH=CHCH2Ph(OH)(OCH3))]4 (1)
2.1.2. Synthesis of G1-[Si(CH2)3NH(CO)CH=CHCH2Ph(OH)2)]4 (2)
2.1.3. Synthesis of G1-[Si(CH2)3NH(CO)Ph(OH)3]4 (3)
2.1.4. Synthesis of G2-[Si(CH2)3NH(CO)CH=CHCH2Ph(OH)(OCH3))]8 (4)
2.1.5. Synthesis of G2-[Si(CH2)3NH(CO)CH=CHCH2Ph(OH)2)]8 (5)
2.1.6. Synthesis of G2-[Si(CH2)3NH(CO)Ph(OH)3]8 (6)
2.2. Spectrophotometric Studies of the Antioxidant Capacity
2.2.1. DPPH Free Radical-Scavenging Activity
2.2.2. FRAP Assay
2.2.3. Analytical Evaluation: Estimation of the IC50 or EC50 and Trolox Equivalent Antioxidant Capacity (TEAC)
2.3. Electrochemical Measurements
2.4. Antibacterial Activity
2.5. Cell Viability
3. Results and Discussion
3.1. Synthesis and Characterization of Polyphenolic Dendrimers
3.2. Antioxidant Capacity Evaluation
3.3. Antibacterial Activity and Viability Assays in HFF-1 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruk, J.; Aboul-Enein, H.Y.; Kładna, A.; Bowser, J.E. Oxidative stress in biological systems and its relation with pathophysiological functions: The effect of physical activity on cellular redox homeostasis. Free Radic. Res. 2019, 53, 497–521. [Google Scholar] [CrossRef] [PubMed]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Milinčić, D.D.; Popović, D.A.; Lević, S.M.; Kostić, A.Ž.; Tešić, Ž.L.; Nedović, V.A.; Pešić, M.B. Application of Polyphenol-Loaded Nanoparticles in Food Industry. Nanomaterials 2019, 9, 1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, R.; Qin, W.; Dai, J.; Zhang, Q.; Lee, K.; Liu, Y. Physicochemical properties of gelatin films containing tea polyphenol-loaded chitosan nanoparticles generated by electrospray. Mater. Des. 2020, 185, 108277. [Google Scholar] [CrossRef]
- Chanphai, P.; Tajmir-Riahi, H.A. Binding analysis of antioxidant polyphenols with PAMAM nanoparticles. J. Biomol. Struct. Dyn. 2018, 36, 3487–3495. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Matei, C.; Deaconu, M.; Stanciuc, A.-M.; Trifan, A.; Gaspar-Pintiliescu, A.; Berger, D. Polyphenols extract from grape pomace. Characterization and valorisation through encapsulation into mesoporous silica-type matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef]
- Lee, C.Y.; Sharma, A.; Cheong, J.E.; Nelson, J.L. Synthesis and antioxidant properties of dendritic polyphenols. Bioorg. Med. Chem. Lett. 2009, 19, 6326–6330. [Google Scholar] [CrossRef] [Green Version]
- Mencía, G.; Sanz del Olmo, N.; Muñoz-Moreno, L.; Maroto-Díaz, M.; Gómez, R.; Ortega, P.; Carmena, M.J.; de la Mata, F.J. Polyphenolic carbosilane dendrimers as anticancer agents against prostate cancer. New J. Chem. 2016, 40, 10488–10497. [Google Scholar] [CrossRef]
- Agawa, H.; Nakazono, M.; Nanbu, S.; Zaitsu, K. Chemiluminescence change of polyphenol dendrimers with different core molecules. Org. Lett. 2008, 10, 5171–5174. [Google Scholar] [CrossRef]
- Jung, D.-I.; Song, J.-H.; Shin, E.-H.; Kim, Y.-Y.; Lee, D.-H.; Choi, S.-K.; Hahn, J.-T. Synthesis of Poly (3, 4, 5-trihydroxybenzoate) dendrimers from Polyphenols and Their Chemiluminescence. Bull. Korean Chem. Soc. 2010, 31, 1031–1034. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.Y.; Nanah, C.N.; Held, R.A.; Clark, A.R.; Huynh, U.G.T.; Maraskine, M.C.; Uzarski, R.L.; McCracken, J.; Sharma, A. Effect of electron donating groups on polyphenol-based antioxidant dendrimers. Biochimie 2015, 111, 125–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrobel, D.; Kłys, A.; Ionov, M.; Vitovic, P.; Waczulikowa, I.; Hianik, T.; Gomez-Ramirez, R.; de la Mata, J.; Klajnert, B.; Bryszewska, M. Cationic carbosilane dendrimers-lipid membrane interactions. Chem. Phys. Lipids 2012, 165, 401–407. [Google Scholar] [CrossRef]
- Carloni, R.; Sanz Del Olmo, N.; Ortega, P.; Fattori, A.; Gómez, R.; Ottaviani, M.F.; García-Gallego, S.; Cangiotti, M.; de la Mata, F.J. Exploring the Interactions of Ruthenium (II) Carbosilane Metallodendrimers and Precursors with Model Cell Membranes through a Dual Spin-Label Spin-Probe Technique Using EPR. Biomolecules 2019, 9, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz del Olmo, N.; Carloni, R.; Bajo, A.M.; Ortega, P.; Fattori, A.; Gómez, R.; Ottaviani, M.F.; García-Gallego, S.; Cangiotti, M.; de la Mata, F.J. Insight into the antitumor activity of carbosilane Cu (ii)–metallodendrimers through their interaction with biological membrane models. Nanoscale 2019, 11, 13330–13342. [Google Scholar] [CrossRef] [PubMed]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Alam, M.A. Anti-hypertensive Effect of Cereal Antioxidant Ferulic Acid and Its Mechanism of Action. Front. Nutr. 2019, 6, 121. [Google Scholar] [CrossRef]
- Kinra, M.; Arora, D.; Mudgal, J.; Pai, K.S.R.; Mallikarjuna Rao, C.; Nampoothiri, M. Effect of Caffeic Acid on Ischemia-Reperfusion-Induced Acute Renal Failure in Rats. Pharmacology 2019, 103, 315–319. [Google Scholar] [CrossRef]
- Khorsandi, K.; Kianmehr, Z.; hosseinmardi, Z.; Hosseinzadeh, R. Anti-cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell Int. 2020, 20, 18. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2019, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- ISO 20776-1:2006. Clinical Laboratory Testing and In Vitro Diagnostic Test Systems–Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Bermejo, J.F.; Ortega, P.; Chonco, L.; Eritja, R.; Samaniego, R.; Müllner, M.; de Jesus, E.; de la Mata, F.J.; Flores, J.C.; Gomez, R. Water-soluble carbosilane dendrimers: Synthesis biocompatibility and complexation with oligonucleotides; evaluation for medical applications. Chem. A Eur. J. 2007, 13, 483–495. [Google Scholar] [CrossRef]
- Chan, L.C.; Cox, B.G. Kinetics of Amide Formation through Carbodiimide/N-Hydroxybenzotriazole (HOBt) Couplings. J. Org. Chem. 2007, 72, 8863–8869. [Google Scholar] [CrossRef]
- Visioli, F.; Bellomo, G.; Galli, C. Free Radical-Scavenging Properties of Olive Oil Polyphenols. Biochem. Biophys. Res. Commun. 1998, 247, 60–64. [Google Scholar] [CrossRef]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Schlesier, K.; Harwat, M.; Böhm, V.; Bitsch, R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Pedulli, G.F.; Cipollone, M. Bond Dissociation Enthalpy of.alpha.-Tocopherol and Other Phenolic Antioxidants. J. Org. Chem. 1994, 59, 5063–5070. [Google Scholar] [CrossRef]
- Blasco, A.J.; González Crevillén, A.; González, M.C.; Escarpa, A. Direct electrochemical sensing and detection of natural antioxidants and antioxidant capacity in vitro systems. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2007, 19, 2275–2286. [Google Scholar] [CrossRef]
- Chevion, S.; Roberts, M.A.; Chevion, M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radic. Biol. Med. 2000, 28, 860–870. [Google Scholar] [CrossRef]
- Ornelas, C.; Ruiz, J.; Belin, C.; Astruc, D. Giant dendritic molecular electrochrome batteries with ferrocenyl and pentamethylferrocenyl termini. J. Am. Chem. Soc. 2009, 131, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Electron-transfer processes in dendrimers and their implication in biology, catalysis, sensing and nanotechnology. Nat. Chem. 2012, 4, 255. [Google Scholar] [CrossRef]
- Zamora, M.; Herrero, S.; Losada, J.; Cuadrado, I.; Casado, C.M.; Alonso, B. Synthesis and electrochemistry of octamethylferrocenyl-functionalized dendrimers. Organometallics 2007, 26, 2688–2693. [Google Scholar] [CrossRef]
- Teixeira, J.; Gaspar, A.; Garrido, E.; Garrido, J.; Borges, F. Hydroxycinnamic Acid Antioxidants: An Electrochemical Overview. BioMed Res. Int. 2013, 2013, 251754. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Paniagua, E.; Manuel Hernandez-Ros, J.; Sanchez-Milla, M.; Alejandra Camero, M.; Maly, M.; Perez-Serrano, J.; Luis Copa-Patino, J.; Sanchez-Nieves, J.; Soliveri, J.; Gomez, R.; et al. Carbosilane cationic dendrimers synthesized by thiol-ene click chemistry and their use as antibacterial agents. RSC Adv. 2014, 4, 1256–1265. [Google Scholar] [CrossRef]







| Polyphenols | N° Polyphenols | Compound | Ep (V) | Ip (µA) | ΔEp (V) |
|---|---|---|---|---|---|
| Ferulic | 1 | L | 0.686 ± 0.009 | 3.70 ± 0.08 | - |
| 4 | G1 (1) | 0.637 ± 0.002 | 0.75 ± 0.06 | - | |
| 8 | G2 (4) | 0.613 ± 0.008 | 0.56 ± 0.02 | - | |
| Caffeic | 1 | L | 0.263 ± 0.005 | 2.91 ± 0.07 | 0.205 ± 0.002 |
| 4 | G1 (2) | 0.276 ± 0.009 | 1.39 ± 0.04 | 0.188 ± 0.006 | |
| 8 | G2 (5) | 0.302 ± 0.010 | 0.143 ± 0.09 | 0.120 ± 0.009 | |
| Gallic | 1 | L | 0.477 ± 0.001 | 3.87 ± 0.08 | - |
| 4 | G1 (3) | 0.410 ± 0.008 | 4.12 ± 0.07 | - | |
| 8 | G2 (6) | 0.409 ± 0.008 | 3.40 ± 0.40 | - |
| Polyphenols | N° Polyphenols | Compound | S. aureus | E. coli | HFF-1 | ||
|---|---|---|---|---|---|---|---|
| MIC80 [ppm] | MIC50 [ppm] | MIC50 [ppm] | [ppm] | % Viability | |||
| Ferulic | 1 | L | >16 | >16 | >16 | - | - |
| 4 | G1 (1) | >16 | >16 | >16 | 16 | 95.4 ± 6.6 | |
| 8 | G2 (4) | >16 | >16 | >16 | 16 | 92.9 ± 7.4 | |
| Caffeic | 1 | L | >16 | >16 | >16 | - | - |
| 4 | G1 (2) | 16 | 8 | >16 | 16 | 100.0 ± 0.0 | |
| 8 | 98.8 ± 1.7 | ||||||
| 8 | G2 (5) | >16 | >16 | >16 | 16 | 100.0 ± 0.0 | |
| Gallic | 1 | L | >16 | >16 | >16 | - | - |
| 4 | G1 (3) | 8 | 4 | 16 | 8 | 99.1 ± 1.0 | |
| 4 | 84.8 ± 7.5 | ||||||
| 8 | G2 (6) | 16 | 16 | >16 | 16 | 100.0 ± 0.0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz del Olmo, N.; Peña González, C.E.; Rojas, J.D.; Gómez, R.; Ortega, P.; Escarpa, A.; de la Mata, F.J. Antioxidant and Antibacterial Properties of Carbosilane Dendrimers Functionalized with Polyphenolic Moieties. Pharmaceutics 2020, 12, 698. https://doi.org/10.3390/pharmaceutics12080698
Sanz del Olmo N, Peña González CE, Rojas JD, Gómez R, Ortega P, Escarpa A, de la Mata FJ. Antioxidant and Antibacterial Properties of Carbosilane Dendrimers Functionalized with Polyphenolic Moieties. Pharmaceutics. 2020; 12(8):698. https://doi.org/10.3390/pharmaceutics12080698
Chicago/Turabian StyleSanz del Olmo, Natalia, Cornelia E. Peña González, Jose Daniel Rojas, Rafael Gómez, Paula Ortega, Alberto Escarpa, and Francisco Javier de la Mata. 2020. "Antioxidant and Antibacterial Properties of Carbosilane Dendrimers Functionalized with Polyphenolic Moieties" Pharmaceutics 12, no. 8: 698. https://doi.org/10.3390/pharmaceutics12080698