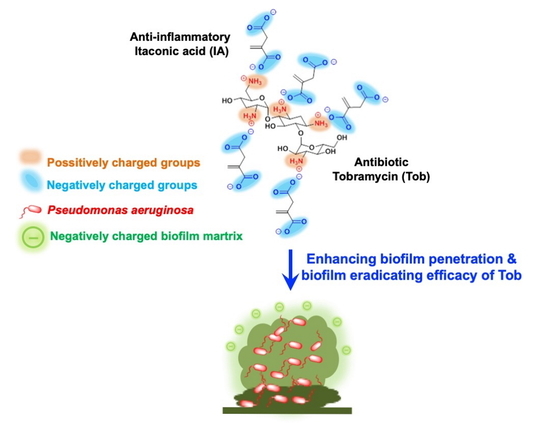
Itaconic Acid Increases the Efficacy of Tobramycin against Pseudomonas aeruginosa Biofilms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Bacteria
2.2. Test Samples Preparation
2.3. Minimum Inhibitory Concentration (MIC) Assay
2.4. Pyocyanin Assay
2.5. Minimum Biofilm Eradicating Concentration (MBEC) Assay
2.6. Tobramycin Diffusion Studies
2.7. Tobramycin Quantification by LC-MS/MS
2.8. Statistical Analysis
3. Results
3.1. Minimum Inhibitory Concentration (MIC) Assay
3.2. Pyocyanin Assay
3.3. Minimum Biofilm Eradicating Concentration (MBEC) Assay
3.4. Tobramycin Diffusion Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ho, D.-K.; Nichols, B.L.B.; Edgar, K.J.; Murgia, X.; Loretz, B.; Lehr, C.-M. Challenges and strategies in drug delivery systems for treatment of pulmonary infections. Eur. J. Pharm. Biopharm. 2019, 144, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: a common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2011, 13, 42–51. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Kioumis, I.; Porpodis, K.; Spyratos, D.; Tsakiridis, K.; Huang, H.; Li, Q.; Turner, J.F.; Browning, R.; Hohenforst-Schmidt, W.; et al. Clinical experimentation with aerosol antibiotics: Current and future methods of administration. Drug Des. Devel. Ther. 2013, 7, 1115–1134. [Google Scholar] [CrossRef] [Green Version]
- Flume, P.A.; VanDevanter, D.R. Clinical applications of pulmonary delivery of antibiotics. Adv. Drug Deliv. Rev. 2015, 85, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef]
- Ho, D.K.; Costa, A.; De Rossi, C.; De Souza Carvalho-Wodarz, C.; Loretz, B.; Lehr, C.M. Polysaccharide Submicrocarrier for Improved Pulmonary Delivery of Poorly Soluble Anti-infective Ciprofloxacin: Preparation, Characterization, and Influence of Size on Cellular Uptake. Mol. Pharm. 2018, 15, 1081–1096. [Google Scholar] [CrossRef]
- Yasar, H.; Ho, D.K.; De Rossi, C.; Herrmann, J.; Gordon, S.; Loretz, B.; Lehr, C.M. Starch-chitosan polyplexes: A versatile carrier system for anti-infectives and gene delivery. Polymers (Basel) 2018, 10, 252. [Google Scholar] [CrossRef] [Green Version]
- Ho, D.-K.; Murgia, X.; De Rossi, C.; Christmann, R.; Hüfner de Mello Martins, A.G.; Koch, M.; Andreas, A.; Herrmann, J.; Müller, R.; Empting, M.; et al. Squalenyl Hydrogen Sulfate Nanoparticles for Simultaneous Delivery of Tobramycin and Alkylquinolone Quorum Sensing Inhibitor to Eradicate P. aeruginosa Biofilm Infections. Angew. Chemie Int. Ed. 2020. [Google Scholar] [CrossRef] [Green Version]
- Ho, D.K.; Frisch, S.; Biehl, A.; Terriac, E.; De Rossi, C.; Schwarzkopf, K.; Lautenschlaeger, F.; Loretz, B.; Murgia, X.; Lehr, C.M. Farnesylated Glycol Chitosan as a Platform for Drug Delivery: Synthesis, Characterization and Investigation of Mucus-Particle Interactions. Biomacromolecules 2018, 19, 3499–3501. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.A.M.; Petrera, L.; Eberhard, J.; Hartmann, R.W. Structure-functionality relationship and pharmacological profiles of: Pseudomonas aeruginosa alkylquinolone quorum sensing modulators. Org. Biomol. Chem. 2017, 15, 4620–4630. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef] [Green Version]
- Bandara, H.M.H.N.; Herpin, M.J.; Kolacny, D.; Harb, A.; Romanovicz, D.; Smyth, H.D.C. Incorporation of Farnesol Significantly Increases the Efficacy of Liposomal Ciprofloxacin against Pseudomonas aeruginosa Biofilms in Vitro. Mol. Pharm. 2016, 13, 2760–2770. [Google Scholar] [CrossRef] [Green Version]
- Cipolla, D.; Blanchard, J.; Gonda, I. Development of liposomal ciprofloxacin to treat lung infections. Pharmaceutics 2016, 8. [Google Scholar] [CrossRef] [Green Version]
- Bilton, D.; Pressler, T.; Fajac, I.; Clancy, J.P.; Sands, D.; Minic, P.; Cipolli, M.; Galeva, I.; Solé, A.; Quittner, A.L.; et al. Amikacin liposome inhalation suspension for chronic Pseudomonas aeruginosa infection in cystic fibrosis. J. Cyst. Fibros. 2019. [Google Scholar] [CrossRef]
- Aksamit, T.; Bandel, T.J.; Criollo, M.; De Soyza, A.; Elborn, J.S.; Operschall, E.; Polverino, E.; Roth, K.; Winthrop, K.L.; Wilson, R. The RESPIRE trials: Two phase III, randomized, multicentre, placebo-controlled trials of Ciprofloxacin Dry Powder for Inhalation (Ciprofloxacin DPI) in non-cystic fibrosis bronchiectasis. Contemp. Clin. Trials 2017, 58. [Google Scholar] [CrossRef] [Green Version]
- MacLeod, D.L.; Barker, L.M.; Sutherland, J.L.; Moss, S.C.; Gurgel, J.L.; Kenney, T.F.; Burns, J.L.; Baker, W.R. Antibacterial activities of a fosfomycin/tobramycin combination: A novel inhaled antibiotic for bronchiectasis. J. Antimicrob. Chemother. 2009, 64, 829–836. [Google Scholar] [CrossRef] [Green Version]
- MacLeod, D.L.; Velayudhan, J.; Kenney, T.F.; Therrien, J.H.; Sutherland, J.L.; Barker, L.M.; Baker, W.R. Fosfomycin enhances the active transport of tobramycin in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2012, 56, 1529–1538. [Google Scholar] [CrossRef] [Green Version]
- Trapnell, B.C.; McColley, S.A.; Kissner, D.G.; Rolfe, M.W.; Rosen, J.M.; McKevitt, M.; Moorehead, L.; Montgomery, A.B.; Geller, D.E. Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with Pseudomonas airway infection. Am. J. Respir. Crit. Care Med. 2012, 185, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniu, S. Novel inhaled combined antibiotic formulations in the treatment of Pseudomonas aeruginosa airways infections in cystic fibrosis. Expert Rev. Anti. Infect. Ther. 2015, 13, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.C.; Landersdorfer, C.B.; McIntosh, M.P.; Peleg, A.Y.; Hirsch, E.B.; Kirkpatrick, C.M.; Bergen, P.J. Clinically relevant concentrations of fosfomycin combined with polymyxin B, tobramycin or ciprofloxacin enhance bacterial killing of Pseudomonas aeruginosa, but do not suppress the emergence of fosfomycin resistance. J. Antimicrob. Chemother. 2016, 71, 2218–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drenkard, E. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect. 2003, 5, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Cordes, T.; Michelucci, A.; Hiller, K. Itaconic Acid: The Surprising Role of an Industrial Compound as a Mammalian Antimicrobial Metabolite. Annu. Rev. Nutr. 2015, 35, 451–473. [Google Scholar] [CrossRef] [PubMed]
- Strelko, C.L.; Lu, W.; Dufort, F.J.; Seyfried, T.N.; Chiles, T.C.; Rabinowitz, J.D.; Roberts, M.F. Itaconic acid is a mammalian metabolite induced during macrophage activation. J. Am. Chem. Soc. 2011, 133, 16386–16389. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Lukat, P.; Iqbal, A.A.; Saile, K.; Kaever, V.; van den Heuvel, J.; Blankenfeldt, W.; Büssow, K.; Pessler, F. Crystal structure of cis-aconitate decarboxylase reveals the impact of naturally occurring human mutations on itaconate synthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 20644–20654. [Google Scholar] [CrossRef] [Green Version]
- Van Nguyen, T.; Alfaro, A.C. Targeted metabolomics to investigate antimicrobial activity of itaconic acid in marine molluscs. Metabolomics 2019, 15. [Google Scholar] [CrossRef]
- Luan, H.H.; Medzhitov, R. Food Fight: Role of Itaconate and Other Metabolites in Antimicrobial Defense. Cell Metab. 2016, 24, 379–387. [Google Scholar] [CrossRef]
- Sasikaran, J.; Ziemski, M.; Zadora, P.K.; Fleig, A.; Berg, I.A. Bacterial itaconate degradation promotes pathogenicity. Nat. Chem. Biol. 2014, 10, 371–377. [Google Scholar] [CrossRef]
- Riquelme, S.A.; Liimatta, K.; Wong Fok Lung, T.; Fields, B.; Ahn, D.; Chen, D.; Lozano, C.; Sáenz, Y.; Uhlemann, A.C.; Kahl, B.C.; et al. Pseudomonas aeruginosa Utilizes Host-Derived Itaconate to Redirect Its Metabolism to Promote Biofilm Formation. Cell Metab. 2020, 31, 1091–1106.e6. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Storz, M.P.; Maurer, C.K.; Zimmer, C.; Wagner, N.; Brengel, C.; De Jong, J.C.; Lucas, S.; Müsken, M.; Häussler, S.; Steinbach, A.; et al. Validation of PqsD as an anti-biofilm target in pseudomonas aeruginosa by development of small-molecule inhibitors. J. Am. Chem. Soc. 2012, 134, 16143–16146. [Google Scholar] [CrossRef] [PubMed]
- Essar, D.W.; Eberly, L.; Hadero, A.; Crawford, I.P. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: Interchangeability of the two anthranilate synthase and evolutionary implications. J. Bacteriol. 1990, 172, 884–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, J.J.; Stremick, C.A.; Turner, R.J.; Allan, N.D.; Olson, M.E.; Ceri, H. Microtiter susceptibility testing of microbes growing on peg lids: A miniaturized biofilm model for high-throughput screening. Nat. Protoc. 2010, 5, 1236–1254. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An overview. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Walters, M.C.; Roe, F.; Bugnicourt, A.; Franklin, M.J.; Stewart, P.S. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 2003, 47, 317–323. [Google Scholar] [CrossRef] [Green Version]
- LeBel, M. Ciprofloxacin: Chemistry, Mechanism of Action, Resistance, Antimicrobial Spectrum, Pharmacokinetics, Clinical Trials, and Adverse Reactions. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1988, 8, 3–30. [Google Scholar] [CrossRef]
- Brillault, J.; Tewes, F. Control of the lung residence time of highly permeable molecules after nebulization: Example of the fluoroquinolones. Pharmaceutics 2020, 12, 387. [Google Scholar] [CrossRef] [Green Version]
- Suci, P.A.; Mittelman, M.W.; Yu, F.P.; Geesey, G.G. Investigation of ciprofloxacin penetration into Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 1994, 38, 2125–2133. [Google Scholar] [CrossRef] [Green Version]
- Wistrand-Yuen, E.; Knopp, M.; Hjort, K.; Koskiniemi, S.; Berg, O.G.; Andersson, D.I. Evolution of high-level resistance during low-level antibiotic exposure. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013. [Google Scholar]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Schütz, C.; Empting, M. Targeting the Pseudomonas quinolone signal quorum sensing system for the discovery of novel anti-infective pathoblockers. Beilstein J. Org. Chem. 2018, 14, 2627–2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen-Cymberknoh, M.; Kerem, E.; Ferkol, T.; Elizur, A. Airway inflammation in cystic fibrosis: Molecular mechanisms and clinical implications. Thorax 2013, 68, 1157–1162. [Google Scholar] [CrossRef] [Green Version]
- De Rose, V.; Burgel, P.R.; Gaggar, A.; Greene, C. Airway inflammatory/immune responses in COPD and cystic fibrosis. Mediators Inflamm. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Roesch, E.A.; Nichols, D.P.; Chmiel, J.F. Inflammation in cystic fibrosis: An update. Pediatr. Pulmonol. 2018, 53, S30–S50. [Google Scholar] [CrossRef] [Green Version]
- De Boeck, K.; Amaral, M.D. Progress in therapies for cystic fibrosis. Lancet Respir. Med. 2016, 4, 662–674. [Google Scholar] [CrossRef]
- Finkelstein, M.; Gold, H.; Paterno, C.A. Pharmacology of itaconic acid and its sodium, magnesium, and calcium salts. J. Am. Pharm. Assoc. Am. Pharm. Assoc. (Baltim) 1947, 36, 173–179. [Google Scholar] [CrossRef]






| Samples | IC90 Against PA14 wt (µg/mL) |
|---|---|
| Tob | 3.125–6.25 |
| Cipro | 3.125 |
| IA | >300 |
| Tob combined IA (molar [Tob]:[IA] of 1:5) | 3.125–6.25 |
| Cipro combined IA (molar [Cipro]:[IA] of 1:5) | 3.125 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, D.-K.; De Rossi, C.; Loretz, B.; Murgia, X.; Lehr, C.-M. Itaconic Acid Increases the Efficacy of Tobramycin against Pseudomonas aeruginosa Biofilms. Pharmaceutics 2020, 12, 691. https://doi.org/10.3390/pharmaceutics12080691
Ho D-K, De Rossi C, Loretz B, Murgia X, Lehr C-M. Itaconic Acid Increases the Efficacy of Tobramycin against Pseudomonas aeruginosa Biofilms. Pharmaceutics. 2020; 12(8):691. https://doi.org/10.3390/pharmaceutics12080691
Chicago/Turabian StyleHo, Duy-Khiet, Chiara De Rossi, Brigitta Loretz, Xabier Murgia, and Claus-Michael Lehr. 2020. "Itaconic Acid Increases the Efficacy of Tobramycin against Pseudomonas aeruginosa Biofilms" Pharmaceutics 12, no. 8: 691. https://doi.org/10.3390/pharmaceutics12080691